INTRODUCTION
Ventriculoperitoneal shunt (VPS) infections are frequent complications that result in shunt failure in 5-15% of patients.1 Common causative agents include Staphylococcus aureus, Coagulase-negative staphylococcus species, a variety of gram-negative rods, Propionibacterium species, and Enterococcus faecalis. Other less common isolates include Haemophilus influenza type B, Klebsiella pneumoniae, Serratia marcescens, Group B Streptococcus, Streptococcus pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa and Morganella morganii. 2 The mechanism of shunt infection most frequently involves colonization of the shunt by skin flora at the time of surgery or postoperatively via breakdown of the wound. Alternatively, shunt infection can occur due to direct contamination of the peritoneal end of the shunt by gut flora in the setting of bowel perforation or peritonitis.3
We present a rare case of a hydrocephalic patient with a ventriculoperitoneal shunt who developed a Streptococcus pyogenes (Group A Streptococcus, GAS) peritoneal catheterassociated intra-abdominal abscess that occurred several weeks after pharyngitis and scarlet fever. GAS colonizes epithelial surfaces, primarily the throat and skin, but also the vagina and rectum, from where it can cause a wide array of superficial, invasive, and immune-mediated diseases. GAS most commonly causes mild infections such as pharyngitis and impetigo, but is also associated with serious non-suppurative complications such as acute rheumatic fever, acute glomerulonephtritis, and toxic shock syndrome.4 Suppurative complications include peritonsillar/retropharyngeal abscess, sinusitis, meningitis, brain abscess, or thrombosis of intracranial venous sinuses.4 However, to our knowledge this is the first case of a peritoneal shunt abscess due to GAS following pharyngitis and scarlet fever reported in the literature. Only one other case of GAS shunt infection has been reported, however that case involved direct contamination of the cranial shunt site.5
CASE REPORT
Our patient was a 31 year-old woman with a history of holoprosencephaly and VPS dependent congenital hydrocephalus requiring a total of nine previous shunt revisions. Her most recent revision was four years prior to presentation due to fractured shunt tubing in the neck. At that time, she underwent a complete shunt revision. The old distal catheter could not be completely removed due to calcification and scarring and was left in place from the upper chest down to the peritoneal cavity. Her immediate post-operative course from that surgery was uneventful with confirmation of correct shunt placement on x-ray and no evidence of CSF leak.
In late December 2013, she developed a systemic illness characterized by cough, fever, and scarlitiniform rash. A throat culture was unable to be obtained due to limited patient cooperation. She was clinically diagnosed with GAS pharyngitis and scarlet fever and treated with a seven-day course of amoxicillin. The patient’s symptoms did not completely resolve and she was treated with a second 7-day course of amoxicillin for presumed streptococcal pharyngitis in February 2014.
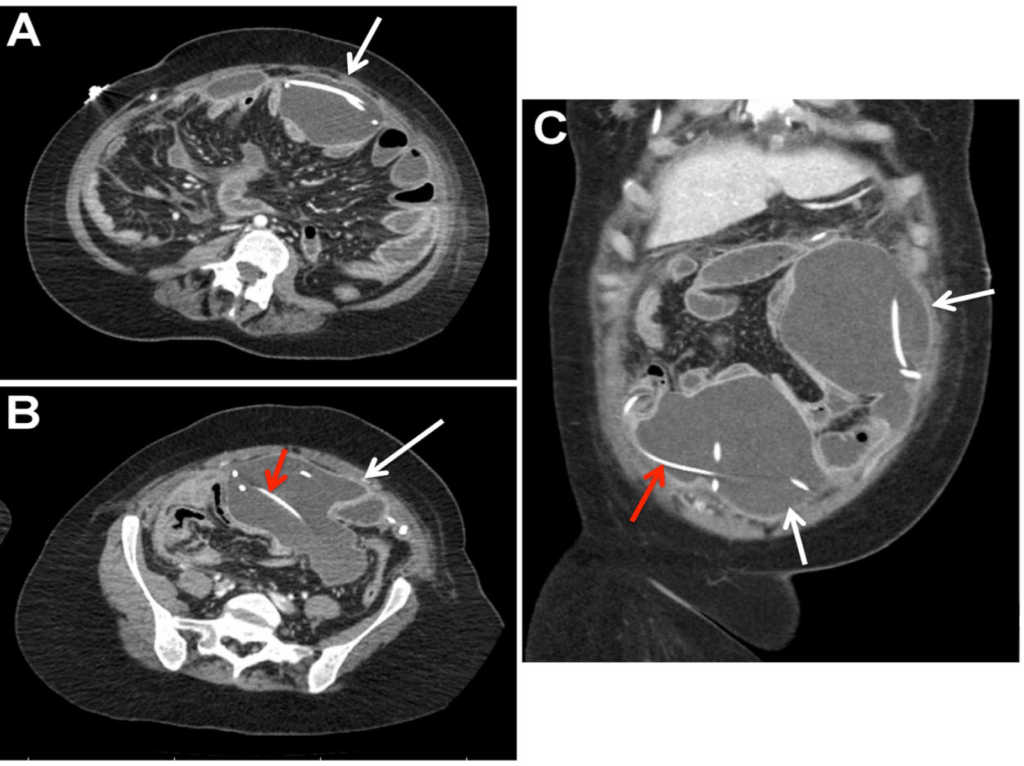
Approximately three weeks later, the patient developed fevers, diaphoresis, diarrhea, and bilious vomiting. She presented to the emergency department at our institution. On physical examination she was febrile to 38.7, tachycardic to the 130s, and with a normal blood pressure. She was at her neurological baseline. Her abdomen was soft, distended, and tender to palpation. Her white blood cell count was elevated, measuring 15.4 x 109 /L with 61% neutrophils and 20% bands. Elevated inflammatory markers were also identified including an erythrocyte sedimentation rate of 103 mm/hr and platelet count of 635 x 109 /L. A Computed Tomography (CT) scan of the abdomen was obtained and revealed a large anterior intra-abdominal loculated fluid collection involving the two VPS peritoneal catheters (Figure 1). CT imaging of her head was unchanged from her previous studies.
Figure 1: Contrast-enhanced computed tomography scan of the abdomen with L5 axial (A), S1 axial (B), and coronal (C) images demonstrating the peritoneal catheters (red arrow) within the large, loculated abdominal abscess (white arrows).
The shunt was immediately externalized at the clavicle and cerebrospinal fluid was evaluated showing: one erythrocyte and no leukocytes per mm3 , glucose of 104 mg/dL, and protein of 10 mg/dL. The patient immediately underwent ultrasound guided percutaneous drainage of the abdominal collection that revealed pus. The resulting cultures of the peritoneal abscess grew numerous colonies of GAS sensitive to penicillin. Blood and CSF cultures showed no growth. Given extensive adhesions and loculations, a laparotomy was required to remove the distal shunt catheters and complete the abscess evacuation.
The patient was treated with a 14-day course of vancomycin given the patient’s known allergy to cephalosporins. After a transthoracic echocardiogram revealed no vegetations, a ventriculoatrial shunt was created to definitively manage her hydrocephalus. This was performed without complications and patient had no subsequent sequalae of her infection.
CONCLUSIONS
Peritoneal GAS shunt-related abscess could be a potential complication of GAS pharyngitis with scarlet fever in a patient with a VPS. Clinicians should be aware of the possibility of hematogenous spread of GAS and VPS infection. A combination of abscess drainage, surgical removal of shunt tubing, and antibiotic therapy was successful in this case. Ventriculoatrial shunting is a reasonable option for CSF diversion when the peritoneum is prohibited due to infection or adhesions.
DISCUSSION
Streptococcus pyogenes is the most common bacterial cause of pharyngitis with over 600 million cases per year.6 Occasionally, GAS pharyngitis is accompanied by scarlet fever, which manifests as a diffuse erythematous and finely papular rash, “strawberry tongue”, and exudative pharyngitis. Development of the scarlet fever rash is thought to be the result of delayed-type skin reactivity to streptococcal pyrogenic exotoxins produced by the organism.4
Less commonly, GAS has the capacity to breach epithelial barriers and cause a variety of invasive diseases. The incidence of severe invasive GAS infections in North America has been estimated to be 1.5 -7.0 cases per 100,000 persons annually.7 Invasive GAS infection is defined as an infection associated with isolation of GAS from a normally sterile body site. Acute peritonitis caused by GAS is a form of invasive infection that is rarely diagnosed in a person without underlying disease such as hepatic cirrhosis, immunosuppression, or nephroticsyndrome.8 In the few cases reported describing GAS peritonitis in healthy women, the entry sites of GAS were not well understood. Ascending genital infections are thought to be the major primary source,8 however other possible primary locations of infection are respiratory tract and cutaneous sites with hematogenous spread of bacteria.9 Direct transluminal spread from the bowel has also been suggested in the pathogenesis of primary GAS peritonitis.10 However, given the preceding pharyngitis in our case, hematogenous spread of GAS to the peritoneum is the most plausible mechanism of infection.
Hematogenous spread as a mode of VPS associated abscess is not well described in the literature. The occurrence of an abdominal abscess following VPS placement is rare, accounting for only 5% of intra-abdominal shunt related complications in one retrospective study.11 The organisms isolated from VPS related abdominal abscesses include pseudomonas,2 staphylococci and enterococcus, 12 consistent with the most common organisms associated with VPS infections. Hepatic abscesses are the most commonly reported intra-abdominal location in the literature. The pathogenic mechanisms postulated are penetration of the shunt tube into the liver and subsequent abscess formation from bacterial translocation from skin flora versus seeding into the liver via portal veins due to intraabdominal sepsis.13 In our patient, the VPS may have served as a nidus for the infection given the large collections surrounding the near entirety of the tubing in the peritoneal cavity.
There has only been one other case of a GAS shunt infection reported in the literature. Patel et al. describe a 7-yearold male who presented with purulent drainage from a cranial shunt site, with GAS isolated from the wound and CSF cultures.5 The mechanism of infection was hypothesized to be autoinoculation from a pharyngeal source as the patient frequently placed his fingers in his mouth and scratched his shunt site. Alternatively, GAS meningitis is a known but rare manifestation of invasive GAS infections, and may have resulted in a secondary shunt infection.14 However, these mechanisms are not likely to be responsible for the shunt infection in our case as the intra-abdominal GAS abscess did not track to the skin and there was no evidence of a CSF infection. Additionally, our patient’s shunt revision surgery was distant enough in the past (4 years prior) that infection from bacterial translocation from skin was unlikely.
The occurrence of a peritoneal shunt infection necessitates shunt externalization and drainage of the infected collection.15 The options for hydrocephalus management thereafter include: replacement of shunt into peritoneum, ventriculoatrial shunt, or placement of shunt tube into pleural cavity. In our case, the recent infection and extensive adhesions within the abdomen excluded the peritoneum as a viable shunt site. A ventriculoatrial shunt was placed for long-term management of hydrocephalus given the patient’s high output of CSF.
ACKNOWLEDGEMENTS
There is no funding source for the work contained in this manuscript.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
CONSENT
Consent for treatment and publication of this report was obtained.







