INTRODUCTION
As to adequate nutritional therapy with metabolic and diabetic patients, the discussion has been continued for long in the light of calorie restriction (CR) and low-carbohydrate diet (LCD).1,2 Several researcher showed the predominant efficacy of LCD compared with CR.3-8 Recently, Bernstein and Feinman have suggested adequate definition and treatment of LCD, in addition to the statement of American Diabetes Association (ADA).1
In Japan, authors and colleagues have treated lots of patients with diabetes mellitus, and investigated the efficacy of LCD.9,10,11 For medical and social development of LCD, we proposed 3 types of actual LCD in daily life, which are petit, standard and super LCD.9,12.13 We have also reported the significant role of ketone bodies in LCD and physiological role for pregnant female and fetus newborn axis.11,14
Furthermore, we investigated the clinical significance of Morbus (M) value in the treatment of LCD for the patients with type 2 diabetes mellitus (T2DM).11,12M-value is useful marker in the evaluation for blood glucose variability in diabetic patients that expresses both elevated glucose level and increased mean amplitude of glycemic excursions (MAGE).15-17 When glycemic control gets better, numerical value of M-value decreases highly, which is simple and helpful for clinical and medical practices and research.
In this study, we have treated T2DM patients for super LCD and investigated the changes of average blood glucose, M-value and renal biomarkers such as uric acid, creatinine and Cystatin C.
SUBJECTS AND METHODS
The subjects were 93 patients with T2DM, including 41 males and 52 females, with 58.3±13.2 years old (mean±SD), and 60 years in median value. They were admitted for 14 days, and received the same treatment protocol for endocrine, metabolic and renal examination.
The methods included formula diet and examination. On admission, CR diet was given on day 1 and 2, including 60% carbohydrates, 25% lipids and 15% protein with 1400 kcal/day. After that, LCD was given from day 3 until day 14, including 12% carbohydrates, 64% lipids and 24% protein with 1400 kcal/ day, which is so-called super LCD formula used for long years in our investigation.9-12
We measured several biomarkers on day 2, 4 and 14. On day 2, the basal biomarkers such as blood glucose/HbA1c, M-value and sera levels of uric acid, creatinine, cystatin C, and daily profile of blood glucose were measured. On day 4, daily profile of blood glucose was measured. On day 14, uric acid, creatinine and other biomarkers were measured.
One subject who is 64-years-old man with HbA1c 7.3% had 3 times of daily profile of glucose. The average glucose and M-value were calculated for that subject.
Analysis for M-value
The M-value (Morbus value) stands for the combination of two factors. One is the level of blood gluose, and another is the MAGE. It is a logarithmic transformation of the deviation of glycemia from an arbitrary assigned “ideal” glucose value.15-17 The formula is as follows: M = MBS+MW, where MW=(maximum blood glucose-minimum glucose)/20; MBS=the mean of MBSBS; MBSBS=individual M-value for each blood glucose value calculated as (absolute value of [10×log (blood glucose value/120)]3 ).
For the M-value, the standard range is <180, borderline is 180-320 and abnormal is >320. Whereas in the M-value, the standard range is <5, borderline is 5-10 and abnormal is >10. It was reported that multiple sampling and a 7-point glycemic trial per day would have yielded similar results.17
Statistical Analyses
In current study, data was represented as the mean±standard deviation, and also represented median, quartile of 25% and 75% in biomarkers. For statistical analyses, correlation coefficients were calculated using the Microsoft Excel analytical tool.18 Furthermore, we used JMP (Version 8) statistical analysis software (JMP Japan Division of SAS Institute Japan Ltd., Minato-ku, Tokyo, Japan) and Microsoft Excel analytical tool. A significance level of less than 5% obtained using a two-tailed test was considered to be statistically significant.
Ethical Considerations
Current study was conducted in compliance with the ethical principles of the Declaration of Helsinki and Japan’s Act on the Protection of Personal Information along with the Ministerial Ordinance on Good Clinical Practice (GCP) for Drug (Ordinance of Ministry of Health and Welfare No. 28 of March 27, 1997). No ethical committee meeting was held. Informed consent was obtained from the subjects related to this research. The study was registered with UMIN #R000031211.
RESULTS
Fundamental data
The results obtained from 93 subjects were shown in Table 1. By the level of M-value, subjects were classified into 4 groups. Group 1-4 revealed the M-value, less than 25, 26-100, 101-250, more than 251, with the number of the cases 24, 24, 24, 21, respectively.
| Table 1: General Data of the Subjects |
|
Categorization
|
Group 1 |
Group 2 |
Group 3 |
Group 4
|
| Number |
24 24
|
24 |
24 |
21
|
| Sex (male/female) |
13/11
|
8/16 |
9/15 |
11/10
|
| Age in average (y.o.) |
56.2±13.3
|
59.1±15.1 |
62.8±7.4 |
54.7±15.1
|
| Age in median (y.o.) |
57 (47-64)
|
63 (50-72) |
63 (58-68) |
60 (50-65)
|
| Body Mass Index (kg/m2 ) |
23.8±3.0
|
24.2±5.2 |
25.4±4.2 |
27.3±5.2
|
| M-value on day 2 |
4-25
|
26-100 |
101-250 |
251-1285
|
The results were expressed by Mean±SD.
The results of age were also expressed by median (25%-75%). |
Glucose Metabolism and M-value
The results for HbA1c, fasting glucose and M-value were shown in Table 2. M-value in 4 groups decreased from day 2 to 4, which was 10.4 to 9.1, 53.5 to 7.7, 150 to 19.1 and 438 to 87, respectively (Figure 1). The average glucose and M-value of 64-years-old patient with T2DM were shown in Table 3.
| Table 2: HbA1c, Glucose and Morbus (M) Value of the Subjects. |
|
Categorization
|
Group 1 |
Group 2 |
Group 3 |
Group 4
|
| HbA1c |
| HbA1c on day 2 in average (%) |
6.6±1.1
|
7.4±1.3 |
8.5±1.2 |
9.5±1.7
|
| HbA1c on day 2 in median (%) |
6.4 (6.1-6.8)
|
7.3 (6.3-8.2) |
8.4 (7.6-9.4) |
9.0 (8.6-10.6) Fasting Glucose
|
| Fasting Glucose |
| Fasting Glucose on day 2 (mg/dL) |
117.3±20.4
|
146.5±31.3 |
183.5±42.5 |
226.5±38.5
|
| Fasting Glucose on day 4 (mg/dL) |
110.5±42.7
|
125.2±27.0 |
147.7±30.6 |
186.8±43.3
|
| Fasting Glucose on day 14 (mg/dL) |
98.9±15.0
|
110.3±21.3 |
119.2±24.4 |
133.1±42.1
|
| Average Glucose |
| average glucose on day 2 (mg/dL) |
128.2±11.8
|
163.6±46.2 |
211.1±20.3 |
298.6±46.3
|
| average glucose on day 4 (mg/dL) |
111.6±19.2
|
135.5±32.2 |
159.0±21.7 |
198.2±47.7
|
| Morbus value |
| M value on day 2 |
10.4 (6.2-17.7)
|
53.5( 41-67) |
150 (125-194) |
438 (343-701)
|
| M value on day 4 |
9.1 (4.4-14.3)
|
7.7 (3.9-19.9) |
19.1 (14.0-29.9) |
87.0 (33-148)
|
The results were expressed by Mean±SD.
The results of HbA1c and M value were expressed by median (25%-75%). |
| Table 3: Average Glucose and M-value of Patient with T2DM. |
|
Blood Glucose level (mg/dL)
|
|
Time (h)
|
8 |
10 |
12 |
14 |
17 |
19 |
21 |
Average |
M-value
|
| Day 2 |
180
|
315 |
288 |
335 |
268 |
333 |
304 |
289 |
426.0
|
| Day 4 |
140
|
191 |
185 |
180 |
152 |
166 |
160 |
168 |
29.0
|
| Day 14 |
97
|
124 |
112 |
125 |
86 |
104 |
93 |
106 |
7.4
|
The patient was 64-years-old male with HbA1c 7.3%.
He was on CR on day 1,2 and LCD on day 3-14.
Blood glucose was measured 7 times a day from 08:00 h to 21:00 h.
M-value stands for combined implication of both average glucose and Mean Amplitude of Glycemic Excursions (MAGE). |
Figure 1: Changes of M-value on Day 2 and 4 in 4 Groups.
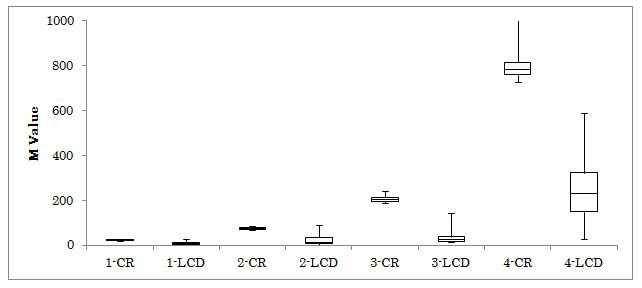
The M-values in 4 groups were investigated on day 2 and 4. The median levels on day 2 to day 4 in 4 groups were 10.4 to 9.1, 53.5 to 7.7, 150 to 19.1 and 438 to 87, respectively. All subjects were provided calorie restricted (CR) diet on day 1 and 2, and low carbohydrate diet (LCD) on day 3 and 4. Abbreviation: 1-CR means group 1 and Calorie Restriction on day 2, and 4-LCD means group 4 and Low Carbohydrate Diet on day 4.
Renal Function
Renal biomarkers on day 2 and day 14 were shown in Table 4. The average uric acid (UA) level in each group revealed significant increase from day 2 to day 14. (Figure 2) There was significant correlation between UA increment and creatinine increment on day 2 and day 14 (Figure 3). Other markers did not show any significant difference in 4 groups.
| Table 4: Renal Function of the Subjects. |
|
Categorization
|
Group 1 |
Group 2 |
Group 3 |
Group 4
|
| Uric Acid |
| Uric Acid on day 2 (mg/dL) |
5.45±1.37
|
4.90±1.46 |
5.33±1.36 |
4.89±1.40
|
| Uric Acid on day 14 (mg/dL) |
6.59±1.92
|
6.19±.2.01 |
6.18±1.29 |
5.90±1.63
|
| Uric Acid increment (mg/dL) |
1.01±1.03
|
1.12±1.02 |
1.22±1.06 |
1.12±1.03
|
| Creatinine |
| Creatinine on day 2 (mg/dL) |
0.75±0.15
|
0.70±0.17 |
0.76±0.21 |
0.64±0.15
|
| Creatinine on day 14 (mg/dL) |
0.81±0.18
|
0.77±0.16 |
0.80±0.23 |
0.72±0.17
|
| Blood Urea Nitrogen |
| BUN on day 2 (mg/dL) |
18.5±5.0
|
18.3±4.8 |
19.3±7.4 |
17.5±4.3
|
| BUN on day 14 (mg/dL) |
22.3±7.2
|
21.2±5.3 |
21.6±11.1 |
17.9±3.8
|
| Other markers |
| Cystatine C on day 2 (mg/L) |
0.81±0.18
|
0.79±0.17 |
0.83±0.32 |
0.69±0.15
|
| CCr on day 4 (ml/min) |
99.7±25.3
|
96.3±29.7 |
95.9±27.2 |
115.3±25.3
|
| eGFR on day 4 (ml/min) |
78.6±19.9
|
75.9±23.4 |
75.6±21.4 |
90.9±19.9
|
| u-uric acid/creatinine |
0.42±0.11
|
0.52±0.18 |
0.53±0.13 |
0.61±0.22
|
| The results were expressed by Mean±SD. |
Figure 2: Changes of Uric Acid on Day 2 and 14 in 4 Groups.
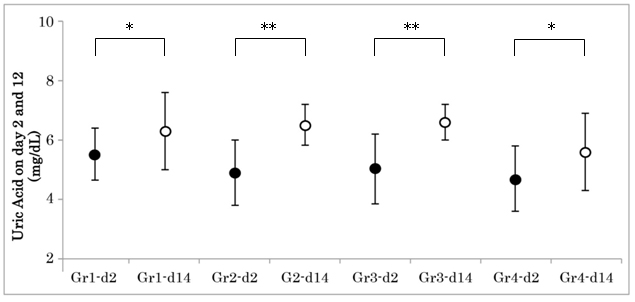
Uric acid (UA) levels were investigated in group 1-4 on day 4 and day 14. The average UA level in each grouprevealed significant increase from day 2 to day 14. The asterisk represents as follows: *p<0.05, **p<0.01. Gr1-d2: Group 1 day 2; Gr4-d14: Group 4 day 14.
Figure 3: Correlation between Uric Acid Increment and Creatinine Increment on Day 2 and Day 14.
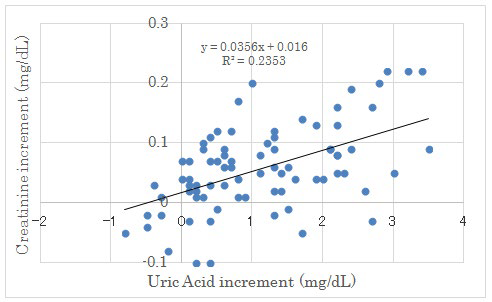
There were significant correlation between both factors (p<0.05).
There was no significant correlation between UA increment from day 2 to 14 and Cystatin C. There was negative significant correlation between Creatinine Clearance (CCr) and Cystatin C. (Figure 4). There was positive significant correlation between Creatinine and Cystatin C (p<0.01) (Figure 5).
Figure 4: Correlation between Creatinine Clearance and Cystatin C.
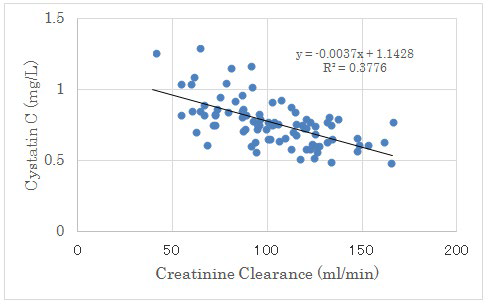
There were negative significant correlation between both factors (p<0.01).
Figure 5: Correlation between Creatinine and Cystatin C.
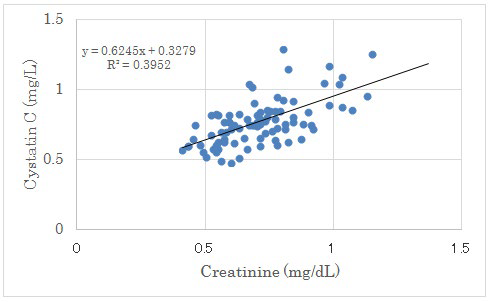
There were positive significant correlation between both factors (p<0.01).
DISCUSSION
LCD was introduced by Bernstein and Atkins, and got developed for long years.19,20 Successively, the effect of LCD have been reported.21-24 In Japan, we have continued clinical study and research concerning LCD, and reported effect of weight reduction, elevated ketone bodies and its physiological role and significant evaluation using M-value.11,12
M-value is a useful biomarker to express the variability of blood glucose including glucose level and fluctuation. There were discussions concerning how many times of sampling per day is necessary. It was reported that multiple sampling and a 7-point glycemic trial per day would have yielded similar results.25-27
M-value reveals similar result for continuous glucose monitoring (CGM) for 48 hours which is considered as ideal research method.28There are rare reports concerning M-value. Patients with T1DM were investigated for blood glucose fluctuations 3 times and more per day, using CGM and self-monitoring of blood glucose (SMBG).29
In this study, the data of M-value and HbA1c in 4 groups showed parallel relation. M-value on day 4 was decreased than that on day 2, suggesting clinical short effect of LCD.
In previous reports, microalbuminuria and glomerular filtration rate (GFR) were followed 14 years, and they did not vary in relation to diabetes status.30,31Continuing LCD with 14% carbohydrate for 1 year, obese adults with T2DM had no adverse affect on clinical markers of renal function or on preexisting kidney disease.32 Similar results were obtained for creatinine, eGFR, albuminuria, or fluid and electrolyte balance.33-34
LCD is as safe as CR and Mediterranean, in preserving/improving renal function among moderately obese participants with or without type 2 diabetes.35 Potential improvement is likely to be mediated by weight loss-induced improvements in insulin sensitivity and blood pressure. For LCD for a year, patients with stage 1-3 renal disease had an improvement in renal function, whereas patients with hyperfiltration had a decrease in the GFR.36
Our current study showed that uric acid was significantly elevated from day 2 to day 14 in 4 groups. One of the causes of elevated uric acid was supposed to be some dehydrated status i.e., less water intake than expected. This is compatible with the result of significant correlation with elevated uric acid and elevated creatinine, with similar elevated tendency of BUN. Another probable cause would be the relative decrease of total calorie intake. When nutrition changes from CR to LCD, total calorie often decreases compared with that of previous status. Third possible cause would be the hyperfiltration of glomerulus in early stage of diabetic nephropathy. As the degree of hyperfiltration becomes less, creatinine and uric acid values could be increased.36 These speculation are possible because of accumulative reports for years.
In comparison with previous reports for years, our study is based on a short period of 2 weeks. Consequently, our speculation would be possibility due to limited research protocol.
In this study, Cystatin C showed significant positive correlation with creatinine and negative correlation with creatinine clearance. Serum Cystatin C alone provides GFR estimates that are nearly as accurate as serum creatinine adjusted for age, sex and race.37The chronic kidney disease-Epidemiology collaboration (CKD-EPI) creatinine equation was reported to be more accurate than the modification of diet in renal disease (MDRD) Study equation.38 Recently, the combined creatinineCystatin C equation have been precise and useful for various patho-physiological states including T2DM.39-41 Our results would become reference data among renal biomarkers in clinical terms which represents actual interrelationship on LCD in the patients with T2DM.
Our study has small and limited research situation, then further research will be necessary concerning the renal functions in LCD.1 LCD would be highly evaluated for treatment of diabetes with the clinical research of influence for renal function.
CONCLUSION
In this study, the changes of M-value on CR/LCD, creatinine, uric acid and Cystatin C were investigated in patients with T2DM. Decreased M indicates the efficacy of LCD for short period, and influence for renal biomarkers in detail relationship will be studied in the future.
ACKNOWLEDGEMENT
The content of this article was presented at the 89th and 90th Scientific Meeting of Japan Endocrine Society (JES) Annual Congress, Kyoto, Japan, 2016 and 2017. The authors would like to thank the patients and staff for their co-operation and support.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.












