INTRODUCTION
The use of computed tomography (CT) has rapidly increased in recent decades as it allows for visualisation of anatomical structures with high spatial and temporal resolution. However, with the extensive amount of CT scans being performed each year, the ionising radiation inherent to CT has become a public concern.1,2 Relative to this, there has been growing interest in radiation dose reduction in CT examinations. Currently, most commercial CT scanners adopt the filtered back-projection (FBP) method to analytically reconstruct images as its process is robust and reconstruction times are quick. However, the image quality of FBP reconstructed images at reduced doses is significantly degraded due to excessive noise levels caused by mathematical assumptions made by the CT system.3,4 To solve these limitations, iterative reconstruction (IR) algorithms were introduced, enabling improved image quality and greater potential for lower radiation doses. However, despite this, the large computational demand and lengthy reconstruction time limits the use of IR techniques.
Recently, researchers have proposed the use of artificial intelligence (AI) to improve CT image reconstruction. One application involves a sharpness-aware general adversarial network to achieve low-dose CT (LDCT) denoising.5 Another concept utilises a multi-scale wavelet domain residual learning architecture for limited-angle CT reconstruction to eliminate artefacts and preserve edges,6 while other approaches involve optimising IR methods through synthetic sinogram-based noise simulations7 or k-sparse autoencoders.8 These AI-based image reconstruction techniques all share a common goal, namely to improve the image quality of low-dose CT images. These methods have shown great promise in achieving exactly this, with several AI algorithms already being clinically implemented. Currently, two CT systems have received 510(k) clearance by the U.S Food and Drug Administration (FDA) for AI-based CT image reconstruction: Advanced intelligent ClearIQ Engine (AiCE), Canon Medical Systems, Tochigi, Japan 9 ) and deep learning (DL) Image Reconstruction (IR)/TrueFidelity™ (GE Healthcare, Illinois, USA10). With the associated advantages of these technologies, it is expected that AI will continue to enhance current reconstruction methods and improve the workflow of clinical CT imaging.
The primary purpose of this literature review is to examine the use of AI-based algorithms in CT reconstruction and its effectiveness in improving the diagnostic image quality of lowdose images. The secondary aims are to provide an overview of the weaknesses of current CT reconstruction methods, namely filtered back-projection and iterative reconstruction, and discuss how machine learning and deep learning algorithms can overcome these limitations.
BACKGROUND
In order to understand the rationale for the use of AI in CT image reconstruction, it is essential to first comprehend the fundamental principles of AI and its subsets of machine learning (ML) and DL. Artificial intelligence refers to a field within computer science whereby an artificial system can mimic human behaviour such as cognitive functions associated with learning capabilities and problem-solving skills.11,12 More recently, advances in both imaging and computers have led to the rapid use of AI in a variety of different radiological applications including characterisation and monitoring of diseases.13
Machine learning is a subset of AI which involves the analysis of complex data sets to learn and find patterns in order to classify categories or predict future conditions without being explicitly programmed.11,12,14,15 ML can be further categorised into supervised and unsupervised learning. In supervised learning, the computer is trained with a dataset that is labelled with ground truth annotations from which the algorithm learns.11,12,14,15,16 This model involves a mathematical relationship between the input data and the labelled outputs, and a predictive model that is validated using unseen test data. Supervised learning is commonly used in two tasks, specifically classification and regression. In classification, the output data is a discrete categorical number whereas in regression, the output data is a continuous numerical value.15,16 In contrast, the algorithm in unsupervised learning learns from unlabelled data which infers an unknown outcome.11,12,14-16 This commonly involves clustering, principal component analysis, and generative adversarial networks (GANs).15,16
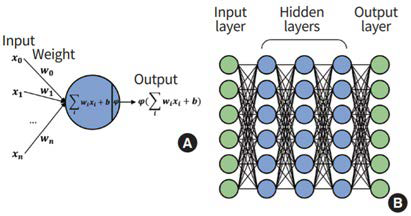
Deep learning is a subset of machine learning that has gained popularity amongst many researchers in recent years. It involves algorithms which contain multiple hidden layers to assess and learn complex patterns within the raw input data.11-13,17 One approach of DL involves an artificial neural network (ANN), which consists of layers with interconnecting nodes including an input layer, a hidden layer responsible for training, and an output layer (Figure 1).16 During training of the ANN, the value of the nodes are updated by parameterising weights through learning algorithms such as back propagation. By iterating the back propagation through the network, optimised weights for each node will reduce losses and the accuracy of the ANN components will be improved.15,16 More recently, ANN has been expanded into deep neural networks (DNN) which incorporates many stacked hidden layers with connecting nodes between the input and output layers. The additional stacked layers have the ability to solve more complex problems by producing simple decisions between the layers. However, despite largely exhibiting improved performance in prediction tasks such as classification and regression, the added layers can cause issues, specifically the vanishing gradient problem and increased computational demand.15,16
Figure 1. Overview of Artificial Neural Network (ANN)
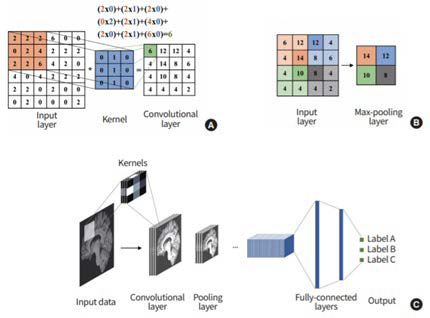
Possibly the most well-known DL architecture is the convolutional neural network (CNN). A CNN comprises a series of convolution, pooling, and fully connecting layers (Figure 2).11,13,15,16,17 The main role of the convolution layer is to recognise and identify patterns in the image. Between the convolution layers are pooling layers which generally extracts the maximum value of the input layer to reduce the size of feature maps and minimise overfitting problems and computational load. These layers are then followed by fully connected layers which combine all activations from the previous layers before an output layer finally provides a prediction.15,16,17
Figure 2. Overview of a Convolutional Neural Network (CNN)
Artificial intelligence, particularly deep learning, has been employed in various medical imaging tasks such as characterisation and monitoring of diseases. However, with the increasing need for radiation dose reductions in CT imaging, the use of AI in CT image reconstruction is now a growing area of interest among researchers.
METHODS
An initial search of the databases Scopus, Ovid MEDLINE, and PubMed was performed for literature published from 2016 to 2020. Only articles that were published within the last four years were included as CT and AI is a quickly evolving area. The search strategy involved keywords which were “artificial intelligence (AI)”, “computed tomography (CT)”, “image reconstruction”, “deep learning (DL)”, and “machine learning (ML)”. A subsequent search of various well-known journals such as Radiology, European Journal of Radiology, Journal of the American College of Radiology, European Radiology, and IEEE Transactions on Medical Imaging was performed to collect additional information on other CT reconstruction techniques such as FBP and IR. The initial search results were screened and assessed for relevance where articles were excluded if they were not obtainable from the databases or were not available in English. Literature that was included in this review either provided general information regarding AI, IR and FBP image reconstruction techniques or were experimental studies that measured the effects of AI-based algorithms on image reconstruction.
RESULTS AND DISCUSSION
For the purpose of this review, it is essential to first comprehend the current CT image reconstruction algorithms and its associated limitations, so as to recognise the need for prospective developments in image reconstruction methods such as the use of artificial intelligence. Various articles provided an overview of the basic principles and limitations of the FBP and IR reconstruction techniques.3,4,18-30.
COMPUTED TOMOGRAPHY RECONSTRUCTION TECHNIQUES
For decades, FBP was the standard image reconstruction method until 2009 when IR techniques were clinically introduced.3 Within a few years, IR methods had quickly progressed into advanced reconstruction algorithms that are still in use today. However, limitations of current reconstruction techniques still exist, providing a rationale for future developments in CT image reconstruction.
Filtered Back Projection
The conventional FBP process involves two main elements: a convolution filter and back-projection. After obtaining the measured projection data, a high-pass filter is applied prior to the linear transformation of the projection data into image space by backprojection.4 This process is repeated with the X-ray source at different angles until the full image has been reconstructed.18 The use of a high-pass kernel is necessary in order to compensate for the effect of blurring that occurs due to the imbalance between the number of projections passing through the centre and periphery of an object.4,18 Utilising a convolution filter reduces the blur and therefore improves the spatial resolution or overall sharpness of the image.4,18
Despite these short reconstruction times and requiring limited computational power, the FBP reconstruction process has various limitations.4 In particular, at reduced doses, FBP is associated with higher image noise and streaking artefacts due to mathematical assumptions made by the CT system.3,20 These approximations include: hardware details such as an infinitely small focal spot size; a pencil-beam X-ray geometry; active detector elements, and X-ray photon statistics such as the poisson distribution.4,19 Additionally, with larger body sizes, photon starvation occurs at low doses resulting in an increase in the susceptibility to artefacts. Hence, with the increasing prevalence of obesity, the image quality of FBP reconstructed images is significantly reduced. As a result of these limitations, IR methods were introduced in order to produce higher quality images at lower radiation doses.3,4
Iterative Reconstruction
IR algorithms provide advantages over FBP, and therefore it is essential to first understand the principles of this technique. Iterative reconstruction algorithms consist of three key steps which first begins with a forward projection of the initial object estimate (derived from the measured projection data) to generate the synthetic projection data. In the second step, a comparison of the artificial raw data with the measured projection data is made to calculate a correction term and lastly through back-projection, the correction term is applied to update the current object estimate. This process is iteratively repeated until a predefined stopping criterion in the object estimate is met or a fixed number of iterations is reached.4,18,20,21
Iterative reconstruction algorithms can be classified into two main categories including statistical (hybrid) and model-base diterative methods. Statistical IR methods, also known as hybrid algorithms combine analytical and iterative techniques in different sequences which either involve the sinogram domain or image domain. In the sinogram, or raw data domain, an edge-preserving denoising algorithm is utilised on the raw projection data in order to minimise image noise and streaking artefacts that would occur without modification.4,18,20 Similarly, image domain algorithms seek to reduce image noise whilst preserving depiction of fine anatomical and low-contrast structures.4,20
Model-based IR (MBIR) algorithms implement models of the acquisition process, image statistics, or system geometry. In general, the more accurate the model to the process, the better the synthetic image can be matched to the raw data and hence lead to improved image quality of the reconstruction. In order to reduce computational complexity and time in MBIR methods, iterative filtration in the sinogram and image domain can be used to reduce the number of iterative projection steps to assist in quicker reconstruction times.4,20,21
Current Examples of Iterative Reconstruction Algorithms
Currently, all major CT vendors have introduced advanced IR algorithms for clinical use in attempt to enable low-dose CT (LDCT) whilst maintaining high image quality. In 2009, Siemens Healthineers was the first vendor to receive FDA clearance for an IR algorithm known as iterative reconstruction in image space (IRIS).3
In the subsequent years, other major vendors such as GE Healthcare, Philips Healthcare, and Canon Healthcare obtained FDA approval for different types of advanced IR algorithms. These techniques can be classified into two categories, namely algorithms that are of image post-processing nature (AIDR3D, IRIS, iDose4, ASIR) and algorithms which can perform one or more iterations through the raw data domain (ASIR-V, Veo, SAFIRE, ADMIRE, IMR, FIRST).22 All CT vendors differ in their approach with the IR process, for example whether they are hybrid-based or modelbased algorithms. However, they all share a common goal aiming for improved noise and artefact reduction with lower reconstruction times in order to enable reduced radiation doses with high diagnostic quality.3,19 A summary of the key features from the current leading IR algorithms offered by major CT vendors is outlined in Table 1.3
| Table 1. Current Leading Iterative Reconstruction Algorithms from the Major CT Vendors3 |
|
CT Vendor
|
Algorithm Name |
Type |
Year of FDA Clearance |
Reconstruction Speed |
Noise Reduction |
Artefact Reduction
|
| GE Healthcare |
ASIR |
Hybrid |
2011 |
Average |
Strong |
Average |
| Veo |
Model-based |
2011 |
Minimal |
Very strong |
Strong |
| Philips Healthcare |
ASIR-V |
Hybrid |
2014 |
Average |
Strong |
Average |
| iDose4 |
Hybrid |
2012 |
Average |
Strong |
Average |
| IMR |
Model-based |
2013 |
Minimal |
Very strong |
Strong |
| Siemens Healthineers |
IRIS |
Hybrid (image domain) |
2009 |
Fast |
Average |
Minimal |
| SAFIRE |
Hybrid |
2011 |
Average |
Strong |
Average |
| ADMIRE |
Model-based |
2012 |
Minimal |
Very strong |
Strong |
| Canon Healthcare |
AIDR3D |
Hybrid |
2012 |
Average |
Strong |
Average |
| FIRST |
Model-based |
2016 |
Minimal |
Very strong |
Strong |
| ASIR: Adaptive statistical image reconstruction; MBIR: Model-based iterative reconstruction; IMR: Iterative model reconstruction; IRIS: Iterative reconstruction in image space; SAFIRE: Sinogram-affirmed iterative reconstruction; ADMIRE: Advanced modelled iterative reconstruction; AIDR3D: Adaptive iterative dose reduction 3D; FIRST: Forward projection model-based iterative reconstruction solution |
Advantages and Limitations of Iterative Reconstruction
Several studies have identified the advantages of IR and its ability to solve limitations inherent to FBP.23-28 For example, the performance in FBP is challenged when reducing the radiation dose due to the mathematical assumptions made by the CT system, however with repeated iterations, a more accurate estimate of the assumptions can be made to create images with decreased noise levels and reduced susceptibility to artefacts.4,19,20 Therefore, in comparison to FBP, IR offers improved image quality with noise and artefact reduction and hence greater potential for lowering radiation doses.3,4,20
Despite the effectiveness of IR methods in enhancing the diagnostic quality of images at reduced radiation doses, limitations still exist. The main weakness of IR algorithms is the increased computational demand and associated slower reconstruction times, particularly for model-based algorithms.4,20 Furthermore, several studies have found that IR techniques are subject to the risk of over smoothing images in addition to reports of unfamiliar image textures such as a “blocky” or “pixelated” appearance around tissue margins.4,18,19,29,30 Relative to these limitations, AI has shown great potential in further improving the reconstruction of CT images.
THE USE OF ARTIFICIAL INTELLIGENCE IN COMPUTED TOMOGRAPHY IMAGE RECONSTRUCTION
There has been an increasing need for radiation dose reductions in CT, however, with the FBP reconstruction method, this results in a trade-off with increased noise levels and lower image quality. Although IR methods are effective in solving the weaknesses of FBP, limitations still arise. Relative to this, artificial intelligence has the potential to overcome the limitations of both FBP and IR reconstruction techniques and further reduce radiation dose levels whilst simultaneously achieving high-quality images.
Application of Artificial Intelligence in Enhancing Image Quality
In recent years, several deep learning-based algorithms have been proposed to enhance image quality in low dose CT images.5,6,31-37 In particular, image noise and artefact susceptibility can be reduced, and structures can be preserved. Chen et al31 proposed the concept of a residual encoder-decoder CNN (RED-CNN) which combined a deconvolutional network, autoencoder, and shortcut connections to achieve LDCT imaging. To validate the performance of the RED-CNN, a dataset of quarter-dose images from the 2016 low-dose CT Grand Challenge was used and comparisons in the root mean square error (RMSE), peak signal-to-noise ratio (PSNR), and structural similarity index (SSIM) were made against five other competing methods. The results demonstrated that the proposed network was highly successful in detail preservation and noise and artefact suppression compared to the other methods.
Cong et al32 proposed an algorithm to accurately match the linear integral model at a target energy level, realise monochromatic imaging, and eliminate beam hardening artefacts. The algorithm was trained using a dataset consisting of dual-energy CT images of the abdomen and performance was tested by inputting conventional CT images into the network to produce corrected projection data. Comparisons were then made between the reconstructed and ground truth images. The results showed that theirmethod has the potential to achieve high accuracy in correcting the projection data with a relative error of less than 0.2%, indicating its effectiveness in improving image quality through monochromatic image reconstruction.
Gu et al6 and Han et al34 both proposed a multi-scale deep residual learning architecture for limited-angle CT reconstruction to eliminate artefacts and preserve structures. Gu and Ye created the algorithm within the wavelet domain, whereas Han et al. developed the network based on a persistent homology analysis. Results showed that the network proposed by Gu and Ye was successful in achieving improved and clear reconstruction with artefact reduction and edge preservation compared to other methods. In terms of quantitative analysis, the proposed network attained the highest PSNR and SSIM, and the lowest NRMSE. Assessments for the performance of the algorithm proposed by Han et al also showed improvements in image reconstruction quality, being most effective in removing streaking artefacts and reducing computational speeds. As for numerical analysis, the network outperformed other methods in PSNR. However, this paper could potentially be improved by using more comparative quantitative metrics such as the SSIM or normalised RMSE like in Gu et al.
Application of Artificial Intelligence in Optimising IR Methods
Besides improving the image quality, AI can be implemented to optimise IR algorithms.7,8,38 Wu et al8 developed an IR method based on priors learned by a k-sparse autoencoder. The algorithm was applied to datasets containing abdominal and chest CT images acquired at different radiation doses. When compared against other widely used priors in terms of SSIM and CNR, the results showed the proposed network to be more successful in noise suppression and structure preservation for quarter-dose data and up to 1/6th of the regular dose.
Another study conducted by Ahn et al7 proposed the use of a deep learning IR method involving a synthetic sinogrambased noise simulation approach to denoise CT images. The first step in training the CNN involved decomposing a noisy sinogram and a noise sinogram to obtain the noise pattern in the sinogram domain. Secondly, the CNN was trained to learn the noise pattern in a supervised manner. Lastly for the test phase, LDCT image denoising was performed by subtracting the noise CT image from the simulated LDCT image to produce a deep IR image. When comparing the LDCT image to the denoised image, quantitative results demonstrated an improvement in the SSIM and PSNR and qualitative results showed that the noise level was reduced by approximately 56%.
Another approach to optimising IR methods was developed by Ziabari et al38 who developed a fast reconstruction algorithm using deep learning to approximate model-based IR (DLMBIR). In addition, they suggested 2D, 2.5D, and 3D variations of their DL-MBIR method. When their methods were applied to clinical datasets, the results demonstrated that the 2.5D DL-MBIR method offered image quality comparable or even better than fully 3D processing with significantly reduced computational cost, indicating the effectiveness of using a 2.5D deep CNN to optimise MBIR techniques.
Overall, machine learning and deep learning approaches have shown great potential in optimising CT image reconstruction, producing promising improvements in image quality and reconstruction times. Table 2 summarises various AI-based CT image reconstruction proposals made by many researchers and their approach in improving and optimising existing reconstruction methods. Currently, the U.S. FDA have approved two AI-based image reconstruction technologies that are now in clinical use.
| Table 2. Summary of Several AI-based CT Image Reconstruction Proposals |
|
References
|
Year |
Purpose |
Proposal
|
| Han et al34 |
2016 |
Artefact reduction Structure preservation Faster computational speeds |
Deep residual learning architecture for sparse-view CT reconstruction based on a persistent homology analysis to remove streaking artefacts and achieve faster computational speeds |
| Chen et al31 |
2017 |
Noise suppression Artefact reduction Structure preservation |
Combining the autoencoder, deconvolution network, and shortcut connections into the residual encoder-decoder convolutional neural network (RED-CNN) for low-dose CT imaging |
| Chen et al36 |
2017 |
Noise suppression Artefact reduction Structure preservation |
Deep CNN to map low-dose CT towards routine-dose CT in a patchby-patch manner |
| Cong et al32 |
2017 |
Artefact reduction |
Deep learning-based reconstruction method to learn an accurate linear integral approach, realise monochromatic imaging, and overcome beam hardening artefacts |
| Du et al37 |
2017 |
Noise suppression Structure preservation |
Deep network architecture known as stacked competitive network (SCN) which comprises several competitive blocks to introduce a multi-scale processing mechanism to further improve the ability of the traditional CNN in suppressing noise and preserving structures |
| Gu et al6 |
2017 |
Artefact reduction Structure preservation |
A multi-scale wavelet-domain residual learning network for limited-angle CT reconstruction to eliminate artefacts and preserve edges |
| Kang et al33 |
2017 |
Noise suppression |
Deep CNN using directional wavelets to detect and remove noise patterns in low-dose CT images |
| Wolterink et al35 |
2017 |
Noise suppression |
Training a CNN together with an adversarial CNN to estimate routine dose from low-dose CT images and hence suppress noise |
| Wu et al8 |
2017 |
Optimise IR methods Noise suppression Structure preservation |
Iterative low-dose CT reconstruction with k-sparse autoencoders (KSAE) trained by artificial neural network |
| Ahn et al7 |
2018 |
Optimise IR methods Noise suppression |
Deep learning IR approach involving a synthetic sinogram-based noise simulation approach for training of convolutional neural network (CNN) to denoise and improve image quality |
| Yi et al5 |
2018 |
Noise suppression |
Applying a sharpness-aware general adversarial network (GAN) to the task of denoising |
| Ziabari et al38 |
2018 |
Optimise IR methods |
Fast reconstruction algorithm using deep learning to approximate model-based IR (DL-MBIR) |
CURRENT ARTIFICIAL INTELLIGENCE – BASED COMPUTED TOMOGRAPHY IMAGE RECONSTRUCTION TECHNOLOGIES
At present, two manufacturers – GE Healthcare and Canon Medical Systems, have received 510(k) clearance by the U.S. FDA for AIbased CT image reconstruction technologies. Compared to current reconstruction methods such as FBP and IR, deep learning-based image reconstruction systemsare able to achieve improved image quality without compromising on dose performance.
Deep Learning Image Reconstruction/TrueFidelity Computed Tomography Images – GE Healthcare
GE Healthcare was the first CT vendor to obtain U.S. FDA clearance for a deep learning image reconstruction (DLIR) technology in April 2019. The DLIR system utilises a deep neural network to generate high quality TrueFidelity™ CT images on their Revolution Apex CT scanner. The main goal of the DLIR engine was to outperform existing CT reconstruction techniques, specifically MBIR methods, in terms of dose performance, image quality, and reconstruction speed.39
To achieve this goal, the system was designed by embedding many layers of mathematical equations and technical knowledge within a DNN. In the training phase, supervised training was performed which involved inputting low dose raw data through the DNN and comparing the output image to a ground-truth image which was a high dose version of the same data. Comparisons of various parameters such as image noise, noise texture, and lowcontrast detectability were made between the low dose and high dose versions of the images. Subsequently, the differences between the images were reported to the DNN network via backpropagation, which was then adjusted to minimise the differences between the images. This training process was then repeated on thousands of training datasets until there was accuracy between the output and ground-truth images. Following the training process came extensive testing whereby the DLIR system was tested to reconstruct many advanced clinical and phantom cases that were not used in the training dataset to ensure its accuracy and robustness.39
In clinical practice, the acquired scan data goes through the DNN-based DLIR engine to generate ground-truth equivalent images, commercially known as TrueFidelity™ CT images. One feature of the DLIR engine is that without affecting the reconstruction speed, three reconstruction strength levels (low, medium, high) can be selected to manage the amount of noise reduction. Assessments of the DLIR system performance was compared against FBP and ASIR-V reconstruction methods. The results demonstrated improved noise reduction efficiency, a noise texture similar to that of high dose FBP, increased contrast-to-noise ratio (CNR), and enhanced low-contrast detectability.39
The resultant TrueFidelity™ CT images evidently improve the image quality compared to FBP and IR image reconstruction methods. Furthermore, it carries great potential for obtaining CT images at reduced dose levels whilst maintaining high diagnostic image quality, which previously limited other CT reconstruction techniques in areas such as low-dose imaging.
Advanced intelligence Clear-IQ Engine Deep Learning Reconstruction–Canon Medical Systems
Canon Medical Systems was the second vendor to receive FDA clearance for a deep learning reconstruction (DLR) technology commercially known as AiCE (advanced intelligence clear-IQ engine). In June 2019, the FDA cleared AiCE for their Aquilion One CT scanner, shortly followed by clearance for the Aquilion Precision system in July 2019, and finally the most recent approval was obtained in February 2020 for the Aquilion Prime SP system. The AiCE engine utilises a deep convolutional neural network to create an algorithm that redefines a balance between high image quality, reconstruction speed, and dose.40
To achieve this, the deep CNN was fitted with a mathematical loss function that determines any errors between the gold standard reference image and the output image. In this case, the reference image was obtained with a high tube current and was reconstructed using MBIR. When comparing the error between the output and reference image, any errors were reported through the network where the weights of the nodes were adjusted in order to correct for the disparities. This process of input-forward and back propagation was repeated until the network could accurately match the output image to that of the gold standard. In order to optimise the accuracy and robustness of AiCE, the training set consisted of millions of image pairs which contained low quality data sets, so that AiCE could learn how to generate high quality images from low quality images whilst maintaining and preserving the signal and spatial resolution. In the validation phase, only low quality datasets that were not included during training were utilised to ensure AiCE could reconstruct high quality images based on what it had learned. This was to reduce overfitting issues and ensure that the algorithm could be widely applicable to clinical practice.40
The AiCE reconstruction process first begins in the sinogram domain where AiCE analyses the raw data and makes modifications. In the projection domain, these modifications improve the signal-to-noise ratio (SNR) and reduces artefacts. The input layer, which is generated from reconstructing the raw data, is then fed into the deep CNN where it is analysed by many hidden convolutional layers. Subsequently, the output of the convolutional layer is fed into a fully connected layer which combines the activations from all the previous layers to determine which node responses will pass to the next layer of the network. After passing through all the hidden layers of the AiCE deep CNN, a signal image which separates the noise from the signal, is generated for the user.40
Evaluations were made for the performance of AiCE DLR in studies of workflow efficiency, low-contrast detectability, noise texture, and spatial resolution. The results demonstrated that AiCE features an image noise texture similar to that of FBP, faster reconstruction times, and improved low-contrast detectability, noise, and spatial resolution relative to hybrid IR.40
The resultant AiCE images undoubtedly demonstrate the effectiveness of the DLR engine in enhancing spatial resolution and low-contrast detectability whilst simultaneously suppressing noise. With reconstruction speeds fast enough for routine clinical use, AiCE seemingly surpasses previous image reconstruction algorithms, enabling high quality CT images at reduced doses.40
Canon Medical Systems was the second vendor to receive FDA clearance for a deep learning reconstruction (DLR) technology commercially known as AiCE (advanced intelligence clear-IQ engine). In June 2019, the FDA cleared AiCE for their Aquilion One CT scanner, shortly followed by clearance for the Aquilion Precision system in July 2019, and finally the most recent approval was obtained in February 2020 for the Aquilion Prime SP system. The AiCE engine utilises a deep convolutional neural network to create an algorithm that redefines a balance between high image quality, reconstruction speed, and dose.40
To achieve this, the deep CNN was fitted with a mathematical loss function that determines any errors between the gold standard reference image and the output image. In this case, the reference image was obtained with a high tube current and was reconstructed using MBIR. When comparing the error between the output and reference image, any errors were reported through the network where the weights of the nodes were adjusted in order to correct for the disparities. This process of input-forward and back propagation was repeated until the network could accurately match the output image to that of the gold standard. In order to optimise the accuracy and robustness of AiCE, the training set consisted of millions of image pairs which contained low quality data sets, so that AiCE could learn how to generate high quality images from low quality images whilst maintaining and preserving the signal and spatial resolution. In the validation phase, only low quality datasets that were not included during training were utilised to ensure AiCE could reconstruct high quality images based on what it had learned. This was to reduce overfitting issues and ensure that the algorithm could be widely applicable to clinical practice.40
The AiCE reconstruction process first begins in the sinogram domain where AiCE analyses the raw data and makes modifications. In the projection domain, these modifications improve the signal-to-noise ratio (SNR) and reduces artefacts. The input layer, which is generated from reconstructing the raw data, is then fed into the deep CNN where it is analysed by many hidden convolutional layers. Subsequently, the output of the convolutional layer is fed into a fully connected layer which combines the activations from all the previous layers to determine which node responses will pass to the next layer of the network. After passing through all the hidden layers of the AiCE deep CNN, a signal image which separates the noise from the signal, is generated for the user.40
Evaluations were made for the performance of AiCE DLR in studies of workflow efficiency, low-contrast detectability, noise texture, and spatial resolution. The results demonstrated that AiCE features an image noise texture similar to that of FBP, faster reconstruction times, and improved low-contrast detectability, noise, and spatial resolution relative to hybrid IR.40
The resultant AiCE images undoubtedly demonstrate the effectiveness of the DLR engine in enhancing spatial resolution and low-contrast detectability whilst simultaneously suppressing noise. With reconstruction speeds fast enough for routine clinical use, AiCE seemingly surpasses previous image reconstruction algorithms, enabling high quality CT images at reduced doses.40
Overall, it is evident that the current commercial deep learning-based CT systems have shown considerable benefits and improvements in terms of image quality which therefore enables further dose reductions. Table 3 outlines the key features of both GE Healthcare’s TrueFidelity™ DLIR engine and Canon Medical System’s AiCE DLR system.
| Table 3. Key Features of GE Healthcare’s TrueFidelity™ DLIR Engine and Canon Medical System’s AiCE DLR Engine39,40 |
|
TrueFidelity™ CT Images – GE HealthCare
|
AiCE– Canon Medical Systems
|
– Image noise reduction
– Noise texture similar to high dose FBP
– Improvement in CNR
– Improved low-contrast detectability |
– Improved low-contrast resolution
– Improved low-contrast detectability, noise and spatial resolution relative to hybrid IR
– Image noise similar to FBP, compared to MBIR – Fast reconstruction (3-5x faster than MBIR) – Easy workflow |
CONCLUSION
Artificial intelligence has been successfully integrated in radiologic tasks such as image recognition, segmentation, and classification. However, this has now been applied to the area of CT image reconstruction as the use of current image reconstruction techniques limits the amount of dose reduction possible without degrading the quality of the image. Therefore, the use of AI is believed to be the next generation image reconstruction option as current FDAapproved technologies have already shown favourable benefits that have markedly impacted healthcare. With further advances, it is expected that AI algorithms will continue to proliferate and not only improve the workflow of CT image reconstruction, but also enable significant reductions in the radiation dose delivered to the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








