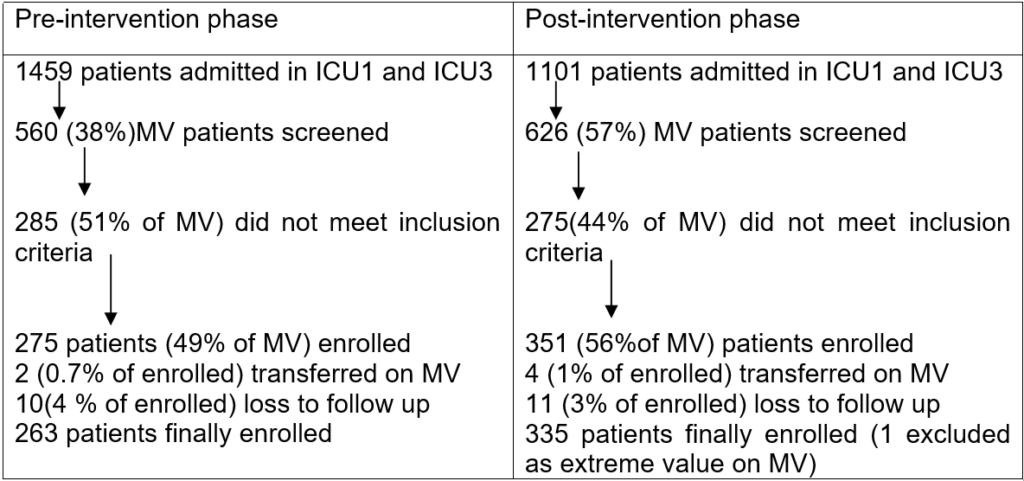
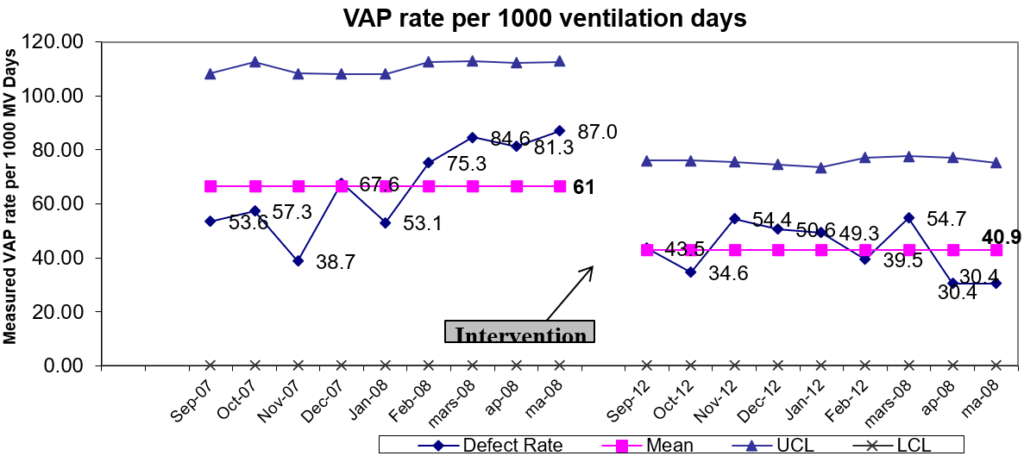
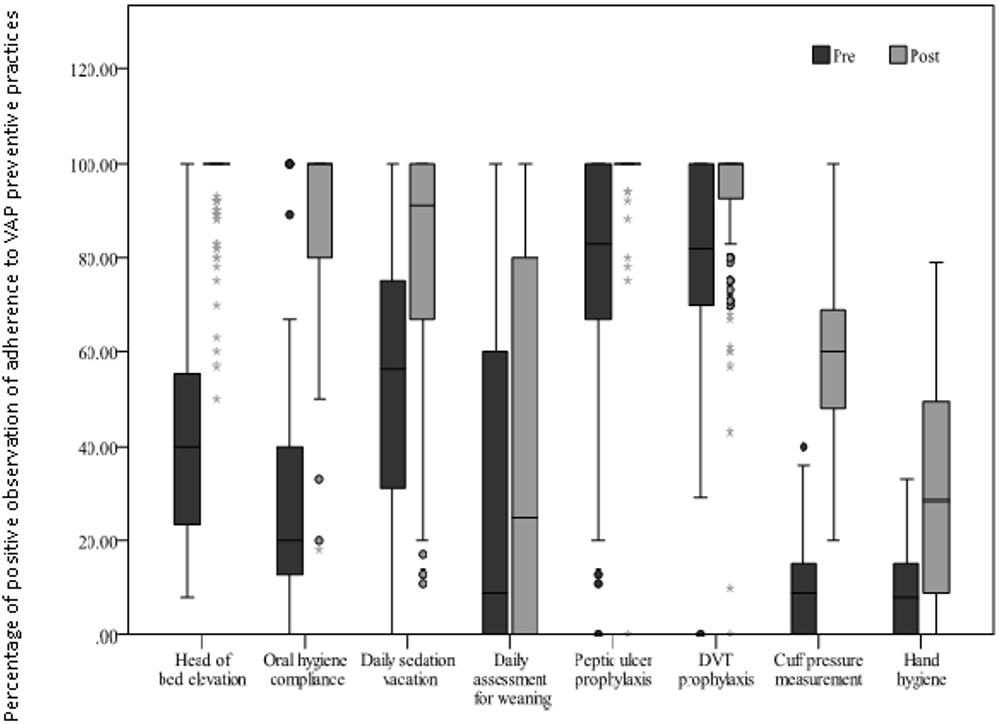
1. Lisboa T, Kollef MH, Rello J. Prevention of VAP: the whole is more than the sum of its parts. Intensive Care Med. 2008; 34(6): 985-987. doi: 10.1007/s00134-008-1101-0
2. Rebmann T, Greene LR. Preventing ventilator-associated pneumonia: An executive summary of the Association for Professionals in Infection Control and Epidemiology, Inc, Elimination Guide. Am J Infect Control. 2010; 38(8): 647-649. doi: 10.1016/j.ajic.2010.08.004
3. Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004; 53(RR-3): 1-36.
4. Dodek P, Keenan S, Cook D, et al. Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004; 141(4): 305-313. doi: 10.7326/0003-4819-141-4-200408170-00011
5. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005; 171(4): 388-416. doi: 10.1164/rccm.200405-644ST
6. El Solh AA, Akinnusi ME, Wiener-Kronish JP, Lynch SV, Pineda LA, Szarpa K. Persistent infection with Pseudomonas aeruginosa in ventilator-associated pneumonia. Am J Respir Crit Care Med. 2008; 178(5): 513-519. doi: 10.1164/rccm.200802-239OC
7. Torres A, Ewig S, Lode H, Carlet J. Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med. 2009; 35(1): 9-29. doi: 10.1007/s00134-008-1336-9
8. Hawe CS, Ellis KS, Cairns CJ, Longmate A. Reduction of ventilator-associated pneumonia: active versus passive guideline implementation. Intensive Care Med. 2009; 35(7): 1180-1186. doi: 10.1007/s00134-009-1461-0
9. Rosenthal VD, Guzman S, Safdar N. Reduction in nosocomial infection with improved hand hygiene in intensive care units of a tertiary care hospital in Argentina. Am J Infect Control. 2005; 33(7): 392-327. doi: 10.1016/j.ajic.2004.08.009
10. Ross A, Crumpler J. The impact of an evidence-based practice education program on the role of oral care in the prevention of ventilator-associated pneumonia. Intensive Crit Care Nurs. 2007; 23(3): 132-136. doi: 10.1016/j.iccn.2006.11.006
11. Arabi Y, Haddad S, Hawes R, et al. Changing sedation practices in the intensive care unit–protocol implementation, multifaceted multidisciplinary approach and teamwork. Middle East J Anesthesiol. 2007; 19(2): 429-447.
12. Jansson M, Kaariainen M, Kyngas H. Effectiveness of educational programmes in preventing ventilator-associated pneumonia: a systematic review. J Hosp Infect. 2013; 84(3): 206-214. doi: 10.1016/j.jhin.2013.04.009
13. Bingham M, Ashley J, De Jong M, Swift C. Implementing a unit-level intervention to reduce the probability of ventilator-associated pneumonia. Nurs Res. 2010 ; 59(1 Suppl): S40-S47. doi: 10.1097/NNR.0b013e3181c3bffc
14. Abbott CA, Dremsa T, Stewart DW, Mark DD, Swift CC. Adoption of a ventilator-associated pneumonia clinical practice guideline. Worldviews Evid Based Nurs. 2006; 3(4): 139-152. doi: 10.1111/j.1741-6787.2006.00066.x
15. Bloos F, Muller S, Harz A, et al. Effects of staff training on the care of mechanically ventilated patients: a prospective cohort study. Br J Anaesth. 2009 ; 103(2): 232-237. doi: 10.1093/bja/aep114
16. Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. Effectiveness of an educational program to reduce ventilator-associated pneumonia in a tertiary care center in Thailand: a 4-year study. Clin Infect Dis. 2007; 45(6): 704-711. doi: 10.1086/520987
17. Zack JE, Garrison T, Trovillion E, et al. Effect of an education program aimed at reducing the occurrence of ventilator-associated pneumonia. Crit Care Med. 2002; 30(11): 2407-2412. doi: 10.1097/00003246-200211000-00001
18. Babcock HM, Zack JE, Garrison T, et al. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Chest. 2004 ; 125(6): 2224-2221. doi: 10.1378/chest.125.6.2224
19. Salahuddina N, Zafarb A, Sukhyanic L, et al. Reducing ventilator-associated pneumonia rates through a staff education programme. Journal of Hospital Infection. 2004; 57: 223-227. doi: 10.1016/j.jhin.2004.03.002
20. Leblebicioglu H, Yalcin AN, Rosenthal VD, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in 11 adult intensive care units from 10 cities of Turkey: findings of the International Nosocomial Infection Control Consortium (INICC). Infection. 2013. doi: 10.1007/s15010-013-0407-1
21. Rosenthal VD, Alvarez-Moreno C, Villamil-Gomez W, et al. Effectiveness of a multidimensional approach to reduce ventilator-associated pneumonia in pediatric intensive care units of 5 developing countries: International Nosocomial Infection Control Consortium findings. Am J Infect Control. 2012; 40(6): 497-501. doi: 10.1016/j.ajic.2011.08.005
22. Guanche-Garcell H, Morales-Perez C, Rosenthal VD. Effectiveness of a multidimensional approach for the prevention of ventilator-associated pneumonia in an adult intensive care unit in Cuba: findings of the International Nosocomial Infection Control Consortium (INICC). J Infect Public Health. 2013; 6(2): 98-107. doi: 10.1097/CCM.0b013e3182657916
23. Tao L, Hu B, Rosenthal VD, Zhang Y, Gao X, He L. Impact of a multidimensional approach on ventilator-associated pneumonia rates in a hospital of Shanghai: findings of the International Nosocomial Infection Control Consortium. J Crit Care. 2012 Oct; 27(5): 440-446. doi: 10.1016/j.jcrc.2011.12.018
24. Rosenthal VD, Pawar M, Leblebicioglu H, et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited-resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013; 34(4): 415-423. doi: 10.1086/669860
25. Coffin SE, Klompas M, Classen D, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals. Infect Control Hosp Epidemiol. 2008; 29 (Suppl 1): S31-S40. doi: 10.1086/591062
26. Craven DE. Preventing ventilator-associated pneumonia in adults: sowing seeds of change. Chest. 2006; 130(1): 251-260 doi: 10.1378/chest.130.1.251
27. Kollef MH. The prevention of ventilator-associated pneumonia. N Engl J Med. 1999; 340: 627-634. doi: 10.1056/NEJM199902253400807
28. Yassi A, Lockhart K, Copes R, et al. Determinants of healthcare workers’ compliance with infection control procedures. Healthc Q. 2007; 10(1): 44-52. doi: 10.12927/hcq.2007.18648
29. Elmenshawy A ET, Abukhaber H, Hafez SF, Ibrahim EH, Fayed AM. Evaluation of the efficacy of a preventive program on ventilator-associated pneumonia. Alexandria Medical Journal. 2010; 52(1): 52-67.
30. Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007 Apr 11; 297(14): 1583-1593. doi: 10.1001/jama.297.14.1583
31. Edwards JR, Peterson KD, Andrus ML, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2006, issued June 2007. Am J Infect Control. 2007; 35(5): 290-301. doi: 10.1016/j.ajic.2007.04.001
32. Jeffrey R, Jacobson GN. Bronchoalveolar lavage. In: Wang KP, Mehta AC, Turner JF, editors. Flexible bronchoscopy. 2nd ed. Massachusetts: Blackwell Science; 2004; 103-115.
33. Gauvin F, Lacroix J, Guertin MC, et al. Reproducibility of blind protected bronchoalveolar lavage in mechanically ventilated children. Am J Respir Crit Care Med. 2002; 165(12): 1618-1623. doi: 10.1164/rccm.2104129
34. Leroy O, Sanders V, Girardie P, et al. Mortality due to ventilator-associated pneumonia: impact of medical versus surgical ICU admittance status. J Crit Care. 2001 Sep; 16(3): 90-97. doi: 10.1053/jcrc.2001.28192
35. Rosenthal VD, Maki DG, Salomao R, et al. Device-associated nosocomial infections in 55 intensive care units of 8 developing countries. Ann Intern Med. 2006; 145(8): 582-591. doi: 10.7326/0003-4819-145-8-200610170-00007
36. Getting started kit: prevent ventilator-associated pneumonia: how-to guide. Crit Care Nurs Q. 2006; 29(2): 157-173. doi: 10.1097/00002727-200604000-00010
37. Leslie E, Geoffrey J, James M. Statistical analysis. Interpretation and uses of medical statistics. 4th ed: Oxford Scientific Publications; 1991; 411-416.
38. Kirkpatrick L, Feeney B, editors. A simple guide to IBM SPSS statistics for version 20.0. Student ed. Belmont, Calif: Wadsworth, Cengage Learning. 2013.
39. Budnitz D, Neuman WR. Underuse of beta-blockers following myocardial infarction. JAMA. 2001; 285(8): 1013. doi: 10.1001/jama.285.8.1013
40. Young MP, Manning HL, Wilson DL, et al. Ventilation of patients with acute lung injury and acute respiratory distress syndrome: has new evidence changed clinical practice? Crit Care Med. 2004; 32(6): 1260-1265. doi: 10.1097/01.ccm.0000127784.54727.56
41. Masterton R, Craven D, Rello J, et al. Hospital-acquired pneumonia guidelines in Europe: a review of their status and future development. J Antimicrob Chemother. 2007; 60(2): 206-213. doi: 10.1093/jac/dkm176
42. Cook D, Ricard JD, Reeve B, et al. Ventilator circuit and secretion management strategies: a Franco-Canadian survey. Crit Care Med. 2000; 28(10): 3547-3554. doi: 10.1097/00003246-200010000-00034
43. Rello J, Lorente C, Bodi M, Diaz E, Ricart M, Kollef MH. Why do physicians not follow evidence-based guidelines for preventing ventilator-associated pneumonia?: a survey based on the opinions of an international panel of intensivists. Chest. 2002; 122(2): 656-661. doi: 10.1378/chest.122.2.656
44. Ricart M, Lorente C, Diaz E, Kollef MH, Rello J. Nursing adherence with evidence-based guidelines for preventing ventilator-associated pneumonia. Crit Care Med. 2003; 31(11): 2693-2696. doi: 10.1097/01.ccm.0000094226.05094.aa
45. Resar R, Pronovost P, Haraden C, Simmonds T, Rainey T, Nolan T. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf. 2005; 31(5): 243-248. doi: 10.1016/s1553-7250(05)31031-2
46. Crunden E, Boyce C, Woodman H, Bray B. An evaluation of the impact of the ventilator care bundle. Nurs Crit Care. 2005; 10(5): 242-246. doi: 10.1111/j.1362-1017.2005.00134.x
47. Burger CD, Resar RK. “Ventilator bundle” approach to prevention of ventilator-associated pneumonia. Mayo Clin Proc. 2006; 81(6): 849-850. doi: 10.4065/81.6.849
48. Fox MY. Toward a zero VAP rate: personal and team approaches in the ICU. Crit Care Nurs Q. 2006; 29(2): 108-114; quiz 15-6. doi: 10.1097/00002727-200604000-00002
49. Lai KK, Baker SP, Fontecchio SA. Impact of a program of intensive surveillance and interventions targeting ventilated patients in the reduction of ventilator-associated pneumonia and its cost-effectiveness. Infect Control Hosp Epidemiol. 2003; 24(11): 859-863. doi: 10.1086/502150
50. Laux L, Herbert C. Decreasing ventilator-associated pneumonia: getting on board. Crit Care Nurs Q. 2006; 29(3): 253-258. doi: 10.1097/00002727-200607000-00011
51. Apostolopoulou E, Bakakos P, Katostaras T, Gregorakos L. Incidence and risk factors for ventilator-associated pneumonia in 4 multidisciplinary intensive care units in Athens, Greece. Respir Care. 2003; 48(7): 681-688.
52. Torres A, Aznar R, Gatell JM, et al. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis. 1990; 142(3): 523-528. doi: 10.1164/ajrccm/142.3.523
53. Erbay RH, Yalcin AN, Zencir M, Serin S, Atalay H. Costs and risk factors for ventilator-associated pneumonia in a Turkish university hospital’s intensive care unit: a case-control study. BMC Pulm Med. 2004; 4: 3. doi: 10.1186/1471-2466-4-3
54. Jarvis WR, Edwards JR, Culver DH, et al. Nosocomial infection rates in adult and pediatric intensive care units in the United States. National Nosocomial Infections Surveillance System. Am J Med. 1991; 91(3B): 185S-191S. doi: 10.1016/0002-9343(91)90367-7
55. Needleman J, Buerhaus P, Mattke S, Stewart M, Zelevinsky K. Nurse-staffing levels and the quality of care in hospitals. N Engl J Med. 2002; 346(22): 1715-1722. doi: 10.1056/NEJMsa012247
56. Cho SH, Ketefian S, Barkauskas VH, Smith DG. The effects of nurse staffing on adverse events, morbidity, mortality, and medical costs. Nurs Res. 2003; 52(2): 71-79. doi: 10.1097/00006199-200303000-00003
57. Salahuddin N, Zafar A, Sukhyani L, et al. Reducing ventilator-associated pneumonia rates through a staff education programme. J Hosp Infect. 2004; 57(3): 223-227. doi: 10.1016/j.jhin.2004.03.002
58. Bouadma L, Deslandes E, Lolom I, et al. Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis. 2010; 51(10): 1115-1122. doi: 10.1086/656737
59. Blamoun J, Alfakir M, Rella ME, et al. Efficacy of an expanded ventilator bundle for the reduction of ventilator-associated pneumonia in the medical intensive care unit. Am J Infect Control. 2009; 37(2): 172-175. doi: 10.1016/j.ajic.2008.05.010
60. Berriel-Cass D, Adkins FW, Jones P, Fakih MG. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Patient Saf. 2006; 32(11): 612-620. doi: 10.1016/s1553-7250(06)32079-x
61. Lansford T, Moncure M, Carlton E, et al. Efficacy of a pneumonia prevention protocol in the reduction of ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt). 2007; 8(5): 505-510. doi: 10.1089/sur.2006.001
62. Rosenthal VD, Guzman S, Crnich C. Impact of an infection control program on rates of ventilator-associated pneumonia in intensive care units in 2 Argentinean hospitals. Am J Infect Control. 2006; 34(2): 58-63. doi: 10.1016/j.ajic.2005.11.002
63. Rosenthal VD, Rodriguez-Calderon ME, Rodriguez-Ferrer M, et al. Findings of the International Nosocomial Infection Control Consortium (INICC), Part II: Impact of a multidimensional strategy to reduce ventilator-associated pneumonia in neonatal intensive care units in 10 developing countries. Infect Control Hosp Epidemiol. 2012; 33(7): 704-710. doi: 10.1086/666342
64. Rosenthal VD, Rodrigues C, Alvarez-Moreno C, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Crit Care Med. 2012; 40(12): 3121-3128. doi: 10.1097/CCM.0b013e3182657916
65. Zilberberg MD, Shorr AF, Kollef MH. Implementing quality improvements in the intensive care unit: ventilator bundle as an example. Crit Care Med. 2009; 37(1): 305-309. doi: 10.1097/CCM.0b013e3181926623
66. Beattie M, Shepherd A, Maher S, Grant J. Continual improvement in ventilator acquired pneumonia bundle compliance: a retrospective case matched review. Intensive Crit Care Nurs. 2012; 28(5): 255-262. doi: 10.1016/j.iccn.2012.01.007
67. Warren DK, Kollef MH. Prevention of hospital infection. Microbes Infect. 2005; 7(2): 268-274. doi: 10.1016/j.micinf.2004.12.003
68. Marra AR, Cal RG, Silva CV, et al. Successful prevention of ventilator-associated pneumonia in an intensive care setting. Am J Infect Control. 2009; 37(8): 619-625. doi: 10.1016/j.ajic.2009.03.009
69. Papadimos TJ, Hensley SJ, Duggan JM, et al. Implementation of the “FASTHUG” concept decreases the incidence of ventilator-associated pneumonia in a surgical intensive care unit. Patient Saf Surg. 2008; 2: 3. doi: 10.1186/1754-9493-2-3
70. Bigham MT, Amato R, Bondurrant P, et al. Ventilator-associated pneumonia in the pediatric intensive care unit: characterizing the problem and implementing a sustainable solution. J Pediatr. 2009; 154(4): 582-587 e2. doi: 10.1016/j.jpeds.2008.10.019
71. Cocanour CS, Peninger M, Domonoske BD, et al. Decreasing ventilator-associated pneumonia in a trauma ICU. J Trauma. 2006; 61(1): 122-129; discussion 9-30. doi: 10.1097/01.ta.0000223971.25845.b3
72. Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med. 2011; 39(10): 2218-2224. doi: 10.1097/CCM.0b013e3182227d52
73. Gastmeier P, Geffers C. Prevention of ventilator-associated pneumonia: analysis of studies published since 2004. J Hosp Infect. 2007; 67(1): 1-8. doi: 10.1016/j.jhin.2007.06.011
74. Omrane R, Eid J, Perreault MM, et al. Impact of a protocol for prevention of ventilator-associated pneumonia. Ann Pharmacother. 2007; 41(9): 1390-1396. doi: 10.1345/aph.1h678
75. Youngquist P, Carroll M, Farber M, et al. Implementing a ventilator bundle in a community hospital. Jt Comm J Qual Patient Saf. 2007; 33(4): 219-225. doi: 10.1016/s1553-7250(07)33026-2
76. Public health focus: surveillance, prevention, and control of nosocomial infections. MMWR Morb Mortal Wkly Rep. 1992; 41(42): 783-787.
77. Jarvis WR. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect Control Hosp Epidemiol. 1996; 17(8): 552-557. doi: 10.1086/647371
78. Boyce JM, Potter-Bynoe G, Dziobek L, Solomon SL. Nosocomial pneumonia in Medicare patients. Hospital costs and reimbursement patterns under the prospective payment system. Arch Intern Med. 1991; 151(6): 1109-1114. doi: 10.1001/archinte.1991.00400060053009
79. Zilberberg MD, Shorr AF. Ventilator-associated pneumonia as a model for approaching cost-effectiveness and infection prevention in the ICU. Curr Opin Infect Dis. 2011; 24(4): 385-389. doi: 10.1097/QCO.0b013e3283474914
80. Klompas M. The paradox of ventilator-associated pneumonia prevention measures. Crit Care. 2009; 13(5): 315. doi: 10.1186/cc8036
81. Chlebicki MP, Safdar N. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2007; 35(2): 595-602. doi: 10.1097/01.ccm.0000253395.70708.ac
82. Drakulovic MB, Torres A, Bauer TT, Nicolas JM, Nogue S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999; 354(9193): 1851-1858. doi: 10.1016/S0140-6736(98)12251-1
83. Edwards JR, Peterson KD, Andrus ML, Dudeck MA, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) Report, data summary for 2006 through 2007, issued November 2008. Am J Infect Control. 2008; 36(9): 609-626. doi: 10.1016/j.ajic.2008.08.001
84. Rosenthal VD, Maki DG, Mehta A, et al. International Nosocomial Infection Control Consortium report, data summary for 2002-2007, issued January 2008. Am J Infect Control. 2008; 36(9): 627-637. doi: 10.1016/j.ajic.2008.03.003
85. Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, Schwab F, et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect. 2006; 64(1): 16-22. doi: 10.1016/j.jhin.2006.04.017
86. (RAISIN) Rsdadiedsdin. Surveillance des infections nosocomiales en re´animation adulte. France, re´sultats 2006, 2007. [cited 2010 5 October]; Available from: http: //www.invs.sante.fr/surveillance/raisin/.
87. Dudeck MA, Horan TC, Peterson KD, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control. 2011; 39(10): 798-816. doi: 10.1016/j.ajic.2011.10.001
88. Geffers C, Gastmeier P. Nosocomial infections and multidrug-resistant organisms in Germany: epidemiological data from KISS (the Hospital Infection Surveillance System). Dtsch Arztebl Int. 2011; 108(6): 87-93. doi: 10.3238/arztebl.2011.0087
89. Skrupky LP, McConnell K, Dallas J, Kollef MH. A comparison of ventilator-associated pneumonia rates as identified according to the National Healthcare Safety Network and American College of Chest Physicians criteria. Crit Care Med. 2012; 40(1): 281-284. doi: 10.1097/CCM.0b013e31822d7913
90. Rosenthal VD, Bijie H, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control. 2012; 40(5): 396-407. doi: 10.1016/j.ajic.2011.05.020
91. Rosenthal VD, Maki DG, Jamulitrat S, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary for 2003-2008, issued June 2009. Am J Infect Control. 2010; 38(2): 95-104 e2. doi: 10.1016/j.ajic.2009.12.004
92. Rosenthal VD, Jarvis WR, Jamulitrat S, et al. Socioeconomic impact on device-associated infections in pediatric intensive care units of 16 limited-resource countries: international Nosocomial Infection Control Consortium findings. Pediatr Crit Care Med. 2012; 13(4): 399-406. doi: 10.1097/PCC.0b013e318238b260
93. Lacherade JC, De Jonghe B, Guezennec P, et al. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010; 182(7): 910-917. doi: 10.1164/rccm.200906-0838OC
94. Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011; 39(8): 1985-1991. doi: 10.1097/CCM.0b013e318218a4d9
95. Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am J Respir Crit Care Med. 2007; 176(11): 1079-1083. doi: 10.1164/rccm.200705-761OC
96. Valles J, Artigas A, Rello J, et al. Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia. Ann Intern Med. 1995; 122(3): 179-186. doi: 10.7326/0003-4819-122-3-199502010-00004
97. Papazian L, Thomas P, Garbe L, et al. Bronchoscopic or blind sampling techniques for the diagnosis of ventilator-associated pneumonia. Am J Respir Crit Care Med. 1995; 152(6(1)): 1982-1991. doi: 10.1164/ajrccm.152.6.8520766
98. Fabregas N, Ewig S, Torres A, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999; 54(10): 867-873. doi: 10.1136/thx.54.10.867
99. Kirtland SH, Corley DE, Winterbauer RH, et al. The diagnosis of ventilator-associated pneumonia: a comparison of histologic, microbiologic, and clinical criteria. Chest. 1997; 112(2): 445-457. doi: 10.1378/chest.112.2.445
100. Arabi Y, Al-Shirawi N, Memish Z, Anzueto A. Ventilator-associated pneumonia in adults in developing countries: a systematic review. Int J Infect Dis. 2008; 12(5): 505-512. doi: 10.1016/j.ijid.2008.02.010
101. Rasslan O, Seliem ZS, Ghazi IA, et al. Device-associated infection rates in adult and pediatric intensive care units of hospitals in Egypt. International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health. 2012; 5(6): 394-402. doi: 10.1016/j.jiph.2012.07.002
102. Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical intensive care units in the United States. Infect Control Hosp Epidemiol. 2000; 21(8): 510-515. doi: 10.1086/501795
103. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36(5): 309-332. doi: 10.1016/j.ajic.2008.03.002
104. Ibrahim EH, Ward S, Sherman G, Kollef MH. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000; 117(5): 1434-1442. doi: 10.1378/chest.117.5.1434
105. Ibrahim EH, Tracy L, Hill C, Fraser VJ, Kollef MH. The occurrence of ventilator-associated pneumonia in a community hospital: risk factors and clinical outcomes. Chest. 2001; 120(2): 555-561. doi: 10.1378/chest.120.2.555