1. Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin SE. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007; 147(2): 227- 235. doi: 10.1111/j.1365-2249.2006.03261.x
2. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014; 15(11): e493-e503. doi: 10.1016/S1470-2045(14)70263-3
3. Crusz SM, Balkwill FR. Inflammation and cancer: Advances and new agents. Nat Rev Clin Oncol. 2015; 12(10): 584-596. doi: 10.1038/nrclinonc.2015.105
4. Lee H, Abston E, Zhang D, Rai A, Jin Y. Extracellular vesicle: An emerging mediator of intercellular crosstalk in lung inflammation and injury. Front Immunol. 2018; 9: 924. doi: 10.3389/fimmu.2018.00924
5. Kunnumakkara AB, Sailo BL, Banik K, et al. Chronic diseases, inflammation, and spices: How are they linked? J Transl Med. 2018; 16(1): 14. doi: 10.1186/s12967-018-1381-2
6. Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M. Ageing: From inflammation to cancer. Immun Ageing. 2018; 15: 1. doi: 10.1186/s12979-017-0112-5
7. Mantovani A. The inflammation – cancer connection. FEBS J. 2018; 285(4): 638-640. doi: 10.1111/febs.14395
8. Martinez BK, White CM. The Emerging role of inflammation in cardiovascular disease. Ann Pharmacother. 2018; 52(8): 801-809. doi: 10.1177/1060028018765939
9. Castaneda S, Gonzalez-Juanatey C, Gonzalez-Gay MA. Inflammation and heart diseases. Curr Pharm Des. 2018; 24(3): 262-280. doi: 10.2174/1381612824666180123102632
10. Wu Y, Dong Y, Duan S, Zhu D, Deng L. Metabolic syndrome, inflammation, and cancer. Mediators Inflamm. 2017; doi: 10.1155/2017/8259356
11. Juergens RA, Wrangle J, Vendetti FP, et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011; 1(7): 598-607. doi: 10.1158/2159-8290.CD-11-0214
12. Al-Talabany S, Mordi I, Graeme Houston J, et al. Epicardial adipose tissue is related to arterial stiffness and inflammation in patients with cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord. 2018; 18(1): 31. doi: 10.1186/s12872-018-0770-z
13. Chen X, Chen X, Xu Y, et al. Association of six CpG-SNPs in the inflammation-related genes with coronary heart disease. Hum Genomics. 2016; 10(2): 21. doi: 10.1186/s40246-016-0067-1
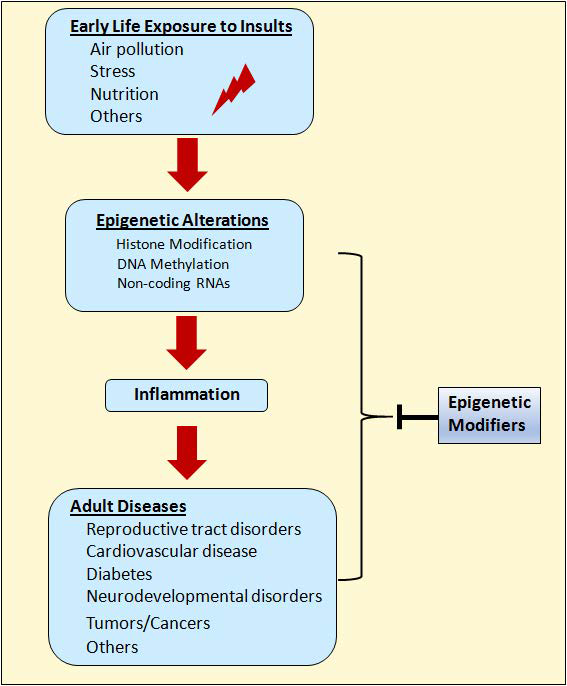
14. Yang Q, Diamond MP, Al-Hendy A. Early life adverse environmental exposures increase the risk of uterine fibroid development: Role of Epigenetic Regulation. Front Pharmacol. 2016; 7: 40. doi: 10.3389/fphar.2016.00040
15. Cook JD, Davis BJ, Cai SL, et al. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc Natl Acad Sci U S A 2005; 102(24): 8644-8649. doi: 10.1073/pnas.0503218102
16. Du Preez A, Leveson J, Zunszain PA, Pariante CM. Inflammatory insults and mental health consequences: Does timing matter when it comes to depression? Psychol Med. 2016; 46(10): 2041- 2057. doi: 10.1017/S0033291716000672
17. Tartaglione AM, Venerosi A, Calamandrei G. Early-life toxic insults and onset of sporadic neurodegenerative diseases-an overview of experimental studies. Curr Top Behav Neurosci. 2016; 29: 231-264. doi: 10.1007/7854_2015_416
18. Spann K, Snape N, Baturcam E, Fantino E. The impact of early-life exposure to air-borne environmental insults on the function of the airway epithelium in asthma. Ann Glob Health. 2016; 82(1): 28-40. doi: 10.1016/j.aogh.2016.01.007
19. Ley D, Desseyn JL, Mischke M, et al. Early-life origin of intestinal inflammatory disorders. Nutr Rev. 2017; 75(3): 175-187. doi: 10.1093/nutrit/nuw061
20. Olvera Alvarez HA, Kubzansky LD, Campen MJ, Slavich GM. Early life stress, air pollution, inflammation, and disease: An integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci Biobehav Rev. 2018; 92: 226- 242. doi: 10.1016/j.neubiorev.2018.06.002
21. Challier JC, Basu S, Bintein T, et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 2008; 29(3): 274-281. doi: 10.1016/j.placenta.2007.12.010
22. Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: The role of maternal obesity. Front Horm Res. 2008; 36: 73-84. doi: 10.1159/0000115355
23. Dunn GA, Bale TL. Maternal high-fat diet effects on thirdgeneration female body size via the paternal lineage. Endocrinology. 2011; 152(6): 2228-2236. doi: 10.1210/en.2010-1461
24. Bolton JL, Bilbo SD. Developmental programming of brain and behavior by perinatal diet: Focus on inflammatory mechanisms. Dialogues Clin Neurosci. 2014; 16(3): 307-320. doi: 10.31887/DCNS.2014.16.3/jbolton
25. Dahlgren J, Nilsson C, Jennische E, et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001; 281(2): E326-E334. doi: 10.1152/ ajpendo.2001.281.2.E326
26. Yang Q, Mas A, Diamond MP, Al-Hendy A. The mechanism and function of epigenetics in uterine leiomyoma development. Reprod Sci. 2016; 23(2): 163-175. doi: 10.1177/1933719115584449
27. Ng GY, Yun-An L, Sobey CG, et al. Epigenetic regulation of inflammation in stroke. Ther Adv Neurol Disord. 2018; 11: 175. doi: 10.1177/1756286418771815
28. Ratay ML, Balmert SC, Bassin EJ, Little SR. Controlled release of an HDAC inhibitor for reduction of inflammation in dry eye disease. Acta Biomater. 2018; 71: 261-270. doi: 10.1016/j. actbio.2018.03.002
29. Tarun A, Antoniades C. The Era of cardiovascular epigenetics: Histone deacetylases and vascular inflammation. Cardiovasc Res.2018; 114(7): 928-930. doi: 10.1093/cvr/cvy099
30. Thankam FG, Boosani CS, Dilisio MF, Agrawal DK. MicroRNAs associated with inflammation in shoulder tendinopathy and glenohumeral arthritis. Mol Cell Biochem. 2018; 437(1-2): 81-97. doi: 10.1007/s11010-017-3097-7
31. Wu XM, Ji KQ, Wang HY, et al. MicroRNA-339-3p alleviates inflammation and edema and suppresses pulmonary microvascular endothelial cell apoptosis in mice with severe acute pancreatitis-associated acute lung injury by regulating Anxa3 via the Akt/ mTOR signaling pathway. J Cell Biochem. 2018; 119(8): 6704-6714. doi: 10.1002/jcb.26859
32. Xia M, Xu H, Dai W, et al. The role of HDAC2 in cigarette smoke-induced airway inflammation in a murine model of asthma and the effect of intervention with roxithromycin. J Asthma. 2018; 55(4): 337-344. doi: 10.1080/02770903.2017.1337788
33. Yang J, Tian B, Brasier AR. Targeting chromatin remodeling in inflammation and fibrosis. Adv Protein Chem Struct Biol. 2017; 107: 1-36. doi: 10.1016/bs.apcsb.2016.11.001
34. Li H, Yao Q, Mariscal AG, et al. Epigenetic control of IL-23 expression in keratinocytes is important for chronic skin inflammation. Nat Commun. 2018; 9(1): 1420. doi: 10.1038/s41467-018- 03704-z
35. Lu L, Claud EC. Intrauterine inflammation, epigenetics, and microbiome influences on preterm infant health. Curr Pathobiol Rep. 2018; 6(1): 15-21. doi: 10.1007/s40139-018-0159-9
36. Wang Q, Trevino LS, Wong RL, et al. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016; 30(8): 856-871. doi: 10.1210/me.2015-1310
37. Jorgensen EM, Alderman MH, Taylor HS. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J. 2016; 30(9): 3194-3201. doi: 10.1096/fj.201500089R
38. Yang Q, Al-Hendy A. Developmental environmental exposure alters the epigenetic features of myometrial stem cells. Gynecol Obstet Res. 2016; 3(2): e1-e4. doi: 10.17140/GOROJ-3-e005
39. Monica F, Elisa P, Caterina S, et al. Changes of intestinal microbiota in early life. J Matern Fetal Neonatal Med. 2018; 10: 1-8. doi: 10.1080/14767058.2018.1506760
40. Park CH, Eun CS, Han DS. Intestinal microbiota, chronic inflammation, and colorectal cancer. Intest Res. 2018; 16(3): 338-345. doi: 10.5217/ir.2018.16.3.338
41. Huh JW, Laurer HL, Raghupathi R, Helfaer MA, Saatman KE. Rapid loss and partial recovery of neurofilament immunostaining following focal brain injury in mice. Exp Neurol. 2002, 175(1): 198-208. doi: 10.1006/exnr.2002.7880
42. Weaver IC, Cervoni N, Champagne FA, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004, 7(8): 847- 854. doi: 10.1038/nn1276
43. Sabry M, Al-Hendy A. Medical treatment of uterine leiomyoma. Reprod Sci. 2012, 19(4): 339-353. doi: 10.1177/1933719111432867
44. Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Pract Res Clin Obstet Gynaecol. 2018; 46: 3-11. doi: 10.1016/j.bpobgyn.2017.09.004
45. Bulun SE. Uterine fibroids. N Engl J Med. 2013; 369(14): 1344- 55. doi: 10.1056/NEJMra1209993
46. Ono M, Qiang W, Serna VA, et al. Role of stem cells in human uterine leiomyoma growth. PLoS One. 2012; 7(5): e36935. doi: 10.1371/journal.pone.0036935
47. Bulun SE, Moravek MB, Yin P, et al. Uterine leiomyoma stem cells: Linking progesterone to growth. Semin Reprod Med. 2015; 33(5): 357-365. doi: 10.1055/s-0035-1558451
48. Mas A, Nair S, Laknaur A, et al. Stro-1/CD44 as putative human myometrial and fibroid stem cell markers. Fertil Steril. 2015; 104(1): 225-234. doi: 10.1016/j.fertnstert.2015.04.021
49. Mas A, Stone L, O’Connor PM, et al. Developmental exposure to endocrine disruptors expands murine myometrial stem cell compartment as a prerequisite to leiomyoma tumorigenesis. Stem Cells. 2017, 35(3): 666-678. doi: 10.1002/stem.2519
50. Alam SR, Stirrat C, Spath N, et al. Myocardial inflammation, injury and infarction during on-pump coronary artery bypass graft surgery. J Cardiothorac Surg. 2017; 12(1): 115. doi: 10.1186/ s13019-017-0681-6
51. Prusinski Fernung LE, Al-Hendy A, Yang Q: A preliminary study: Human fibroid stro-1(+)/CD44(+) stem cells isolated from uterine fibroids demonstrate decreased DNA repair and genomic integrity compared to adjacent myometrial stro-1(+)/ CD44(+) cells. Reprod Sci. 2018; doi: 10.1177/1933719118783252
52. Protic O, Toti P, Islam MS, et al. Possible involvement of inflammatory/reparative processes in the development of uterine fibroids. Cell Tissue Res. 2016; 364(2): 415-427. doi: 10.1007/ s00441-015-2324-3
53. Ciebiera M, Wlodarczyk M, Wrzosek M, et al. TNF-alpha serum levels are elevated in women with clinically symptomatic uterine fibroids. Int J Immunopathol Pharmacol. 2018; 32: 2058738418779461. doi: 10.1177/2058738418779461.
54. Orciani M, Caffarini M, Biagini A, et al. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int. 2018; doi: 10.1155/2018/1716246
55. Yang Q, Trevino L, EI Andaloussi A, et al. Developmental reprogramming of pro-inflammatory pathway mediates adult onset of uterine fibroids. American Society for Reproductive Medcine. 2018.doi: 10.1016/j.fertnstert.2018.07.1053
56. Sadeghi A, Rostamirad A, Seyyedebrahimi S, Meshkani R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology. 2018; doi: 10.1007/s10787-018-0466-0
57. Wang J, Zhao L, Wei Z, et al. Inhibition of histone deacetylase reduces lipopolysaccharide-induced-inflammation in primary mammary epithelial cells by regulating ROS-NF-small ka, CyrillicB signaling pathways. Int Immunopharmacol. 2018, 56: 230-234.doi: 10.1016/j.intimp.2018.01.039
58. Xu Y, Liu L. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-kappaB signaling pathway. Influenza Other Respir Viruses. 2017; 11(5): 457-463. doi: 10.1111/irv.12459
59. Mishra N, Brown DR, Olorenshaw IM, Kammer GM. Trichostatin a reverses skewed expression of CD154, interleukin-10, and interferon-gamma gene and protein expression in lupus T cells. Proc Natl Acad Sci U S A. 2001; 98(5): 2628-2633. doi: 10.1073/ pnas.051507098
60. Yang F, Yang Y, Wang Y, et al. Selective class I histone deacetylase inhibitors suppress persistent spontaneous nociception and thermal hypersensitivity in a rat model of bee venom-induced inflammatory pain. Sheng Li Xue Bao. 2015; 67(5): 447-454.
61. Heers H, Stanislaw J, Harrelson J, Lee MW. Valproic acid as an adjunctive therapeutic agent for the treatment of breast cancer. Eur J Pharmacol. 2018; 835: 61-74. doi: 10.1016/j.ejphar.2018.07.057
62. Hajmirza A, Emadali A, Gauthier A, et al. BET family protein BRD4: An emerging actor in NFkappaB signaling in inflammation and cancer. Biomedicines. 2018; 6(1): E16. doi: 10.3390/biomedicines6010016
63. Shi J, Vakoc CR. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol Cell. 2014; 54(5): 728- 36. doi: 10.1016/j.molcel.2014.05.016
64. Mertz JA, Conery AR, Bryant BM, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011; 108(40): 16669-16674. doi: 10.1073/ pnas.1108190108
65. Chatterjee N, Bohmann D. BET-ting on Nrf2: How Nrf2 signaling can influence the therapeutic activities of BET protein inhibitors. Bioessays. 2018, 40(5): e1800007. doi: 10.1002/ bies.201800007
66. Saenz DT, Fiskus W, Manshouri T, et al. BET protein bromodomain inhibitor-based combinations are highly active against post-myeloproliferative neoplasm secondary AML cells. Leukemia. 2017; 31(3): 678-687. doi: 10.1038/leu.2016.260
67. Dawson MA, Prinjha RK, Dittmann A, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011; 478(7370): 529-533.doi: 10.1038/nature10509
68. Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci U S A. 2012; 109(47): 19408-19413. doi: 10.1073/pnas.1216363109
69. Bid HK, Kerk S. BET bromodomain inhibitor (JQ1) and tumor angiogenesis. Oncoscience. 2016; 3(11-12): 316-317. doi: 10.18632/oncoscience.326
70. Bid HK, Phelps DA, Xaio L, et al. The bromodomain BET inhibitor JQ1 suppresses tumor angiogenesis in models of childhood sarcoma. Mol Cancer Ther. 2016; 15(5): 1018-1028. doi: 10.1158/1535-7163.MCT-15-0567
71. Magistri M, Velmeshev D, Makhmutova M, et al. The BETbromodomain inhibitor JQ1 reduces inflammation and tau phosphorylation at Ser396 in the brain of the 3xTg model of alzheimer’s disease. Curr Alzheimer Res. 2016; 13(9): 985-995. doi: 10.2174/1567205013666160427101832
72. Eskandarpour M, Alexander R, Adamson P, Calder VL. Pharmacological inhibition of bromodomain proteins suppresses retinal inflammatory disease and downregulates retinal Th17 cells. J Immunol. 2017; 198(3): 1093-1103. doi: 10.4049/jimmu nol.1600735
73. Cheung K, Lu G, Sharma R, et al. BET N-terminal bromodomain inhibition selectively blocks Th17 cell differentiation and ameliorates colitis in mice. Proc Natl Acad Sci U S A. 2017; 114(11): 2952-2957. doi: 10.1073/pnas.1615601114
74. Manara MC, Valente S, Cristalli C, et al. A quinoline-based DNA methyltransferase inhibitor as a possible adjuvant in osteosarcoma therapy. Mol Cancer Ther. 2018; 17(9): 1881-1892. doi: 10.1158/15357163.MCT-17-0818
75. Yang J, Tian X, Yang J, et al. 5-Aza-2’-deoxycytidine, a DNA methylation inhibitor, induces cytotoxicity, cell cycle dynamics and alters expression of DNA methyltransferase 1 and 3A in mouse hippocampus-derived neuronal HT22 cells. J Toxicol Environ Health A. 2017; 80(22): 1222-1229. doi: 10.1080/15287394.2017.1367143
76. Andrade AF, Borges KS, Suazo VK, et al. The DNA methyltransferase inhibitor zebularine exerts antitumor effects and reveals BATF2 as a poor prognostic marker for childhood medulloblastoma. Invest New Drugs. 2017; 35(1): 26-36. doi: 10.1007/s10637-016-0401-4
77. Shilpi A, Parbin S, Sengupta D, et al. Mechanisms of DNA methyltransferase-inhibitor interactions: Procyanidin B2 shows new promise for therapeutic intervention of cancer. Chem Biol Interact. 2015; 233: 122-138. doi: 10.1016/j.cbi.2015.03.022
78. Konac E, Varol N, Yilmaz A, Menevse S, Sozen S. DNA methyltransferase inhibitor-mediated apoptosis in the Wnt/ beta-catenin signal pathway in a renal cell carcinoma cell line. Exp Biol Med (Maywood). 2013; 238(9): 1009-1016. doi: 10.1177/1535370213498984
79. Yang QW, Liu S, Tian Y, et al. Methylation-associated silencing of the thrombospondin-1 gene in human neuroblastoma. Cancer Res. 2003; 63(19): 6299-6310.
80. Griffiths EA, Gore SD. DNA methyltransferase and histone deacetylase inhibitors in the treatment of myelodysplastic syndromes. Semin Hematol. 2008; 45(1): 23-30. doi: 10.1053/j.seminhematol.2007.11.007
81. Singer BD, Mock JR, Aggarwal NR, et al. Regulatory T cell DNA methyltransferase inhibition accelerates resolution of lung inflammation. Am J Respir Cell Mol Biol. 2015; 52(5): 641-652. doi: 10.1165/rcmb.2014-0327OC
82. Wang J, Hodes GE, Zhang H, et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat Commun. 2018; 9(1): 477. doi: 10.1038/s41467- 017-02794-5
83. Adams KR, Chauhan S, Patel DB, et al. Ubiquitin Conjugation Probed by Inflammation in Myeloid-Derived Suppressor Cell Extracellular Vesicles. J Proteome Res. 2018; 17(1): 315-324. doi: 10.1021/acs.jproteome.7b00585
84. Hammitzsch A, Tallant C, Fedorov O, et al. CBP30, a selective CBP/p300 bromodomain inhibitor, suppresses human Th17 responses. Proc Natl Acad Sci U S A. 2015; 112(34): 10768-10773. doi: 10.1073/pnas.1501956112
85. Thangavel J, Samanta S, Rajasingh S, et al. Epigenetic modifiers reduce inflammation and modulate macrophage phenotype during endotoxemia-induced acute lung injury. J Cell Sci. 2015; 128(16): 3094-3105. doi: 10.1242/jcs.170258
86. Shen J, Wu S, Guo W, et al. Epigenetic regulation of proinflammatory cytokine genes in lipopolysaccharide -stimulated peripheral blood mononuclear cells from broilers. Immunobiology. 2017; 222(2): 308-315. doi: 10.1016/j.imbio.2016.09.009
87. Grabiec AM, Krausz S, de Jager W, et al. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J Immunol. 2010; 184(5): 2718-2728. doi: 10.4049/jimmunol.0901467
88. Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011; 17(5-6): 391-396. doi: 10.2119/molmed.2011.00058
89. Leoni F, Fossati G, Lewis EC, et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med. 2005; 11(1-12): 1-15. doi: 10.2119/2006-00005.Dinarello
90. Leoni F, Zaliani A, Bertolini G, et al. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002; 99(5): 2995-3000. doi: 10.1073/pnas.052702999
91. Gillespie J, Savic S, Wong C, et al. Histone deacetylases are dysregulated in rheumatoid arthritis and a novel histone deacetylase 3-selective inhibitor reduces interleukin-6 production by peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Rheum. 2012; 64(2): 418-422. doi: 10.1002/art.33382
92. Saouaf SJ, Li B, Zhang G, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp Mol Pathol. 2009; 87(2): 99-104. doi: 10.1016/j.yexmp.2009.06.003
93. Marquardt JU, Gomez-Quiroz L, Arreguin Camacho LO, et al. Curcumin effectively inhibits oncogenic NF-kappaB signaling and restrains stemness features in liver cancer. J Hepatol. 2015; 63(3): 661-669. doi: 10.1016/j.jhep.2015.04.018
94. Chen Y, Shu W, Chen W, et al. Curcumin, both histone deacetylase and p300/CBP-specific inhibitor, represses the activity of nuclear factor kappa B and Notch 1 in Raji cells. Basic Clin Pharmacol Toxicol. 2007; 101(6): 427-433. doi: 10.1111/j.1742- 7843.2007.00142.x
95. Wei ZQ, Zhang YH, Ke CZ, et al. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J Gastroenterol. 2017; 23(34): 6252-6260. doi: 10.3748/wjg.v23.i34.6252
96. Kadiyala CS, Zheng L, Du Y, et al. Acetylation of retinal histones in diabetes increases inflammatory proteins: effects of minocycline and manipulation of histone acetyltransferase (HAT) and histone deacetylase (HDAC). J Biol Chem. 2012; 287(31): 25869-25880. doi: 10.1074/jbc.M112.375204
97. Busbee PB, Nagarkatti M, Nagarkatti PS. Natural indoles, indole-3-carbinol and 3,3’-diindolymethane, inhibit T cell activation by staphylococcal enterotoxin B through epigenetic regulation involving HDAC expression. Toxicol Appl Pharmacol. 2014; 274(1): 7-16. doi: 10.1016/j.taap.2013.10.022
98. Nishida K, Komiyama T, Miyazawa S, et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004; 50(10): 3365-3376. doi: 10.1002/ art.20709
99. Zhang ZY, Schluesener HJ. HDAC inhibitor MS-275 attenuates the inflammatory reaction in rat experimental autoimmune prostatitis. Prostate. 2012; 72(1): 90-99. doi: 10.1002/pros.21410
100. Hogh Kolbaek Kjaer AS, Brinkmann CR, Dinarello CA, et al. The histone deacetylase inhibitor panobinostat lowers biomarkers of cardiovascular risk and inflammation in HIV patients. AIDS. 2015; 29(10): 1195-1200. doi: 10.1097/QAD.0000000000000678
101. Qu X, Proll M, Neuhoff C, et al. Sulforaphane epigenetically regulates innate immune responses of porcine monocyte-derived dendritic cells induced with lipopolysaccharide. PLoS One. 2015; 10(3): e0121574. doi: 10.1371/journal.pone.0121574
102. Orecchia A, Scarponi C, Di Felice F, et al. Sirtinol treatment reduces inflammation in human dermal microvascular endothelial cells. PLoS One. 2011; 6(9): e24307. doi: 10.1371/journal. pone.0024307
103. Lugrin J, Ciarlo E, Santos A, et al. The sirtuin inhibitor cambinol impairs MAPK signaling, inhibits inflammatory and innate immune responses and protects from septic shock. Biochim Biophys Acta. 2013; 1833(6): 1498-1510. doi: 10.1016/j.bbamcr.2013.03.004
104. Cantley MD, Fairlie DP, Bartold PM, et al. Inhibiting histone deacetylase 1 suppresses both inflammation and bone loss in arthritis. Rheumatology (Oxford). 2015; 54(9): 1713-1723. doi: 10.1093/rheumatology/kev022
105. Zhang ZY, Schluesener HJ. Oral administration of histone deacetylase inhibitor MS-275 ameliorates neuroinflammation and cerebral amyloidosis and improves behavior in a mouse model. J Neuropathol Exp Neurol. 2013; 72(3): 178-185. doi: 10.1097/NEN.0b013e318283114a
106. Zhang ZY, Zhang Z, Schluesener HJ. MS-275, an histone deacetylase inhibitor, reduces the inflammatory reaction in rat experimental autoimmune neuritis. Neuroscience. 2010; 169(1): 370- 377. doi: 10.1016/j.neuroscience.2010.04.074
107. Vishwakarma S, Iyer LR, Muley M, et al. Tubastatin, a selective histone deacetylase 6 inhibitor shows anti-inflammatory and anti-rheumatic effects. Int Immunopharmacol. 2013; 16(1): 72-78. doi: 10.1016/j.intimp.2013.03.016