INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a common and progressive respiratory disease characterized by airflow limitation that is not fully reversible. It is associated with a high-level of morbidity and mortality, particularly in those with more severe disease. The World Health Organization (WHO) estimates that COPD will become the third leading cause of death worldwide by 2030, with approximately 5% of the global population affected by the disease.1 The disease is often accompanied by a wide range of symptoms, including dyspnea, cough, and sputum production limiting physical activity and social interactions and negatively impacting patients’ quality of life. It also places a considerable economic burden on healthcare systems, with COPD-related hospitalizations and medication costs accounting for a significant proportion of healthcare expenditure.2
The development of effective treatments for COPD is therefore of great importance. Inhaled bronchodilators and corticosteroids are the mainstay of pharmacological treatment for COPD, and there is growing evidence for the benefits of combining these agents to achieve greater symptom control and disease management.3 However, the use of inhalants comes with a range of challenges, which undermines their effective clinical application, including poor inhaler application technique, drug interactions, side effects, and drug resistance. Hence, the quest for developing complementary treatments that can slow disease progression, reduce exacerbations, and improve long-term outcomes for patients with COPD is ongoing.
In recent years, several new drugs and combination therapies have been developed for COPD, including triple combination therapies, as well as a range of add-on treatments. Add-on treatments for COPD are marketed in two categories A. prescription drugs, e.g., phosphodiesterase-4 inhibitors and monoclonal antibodies, and B. OTC supplements. The indications and acceptability of the latter have not been sufficiently established among practicing clinicians due to a lack of research attention. Nonetheless, some evidence for the clinical benefits of certain natural compounds/nutrients on COPD outcomes is available, among these are vitamin D, minerals, omega-3 fatty acids, and antioxidants.4 Exploring the role of nutrients in the management of COPD patients is important given the fact that this group of individuals often suffer from nutritional deficiencies due to reduced dietary intake, increased energy expenditure, and systemic inflammation among others.5
LithoLexal® Respiro is a first-in-class oral add-on-therapy for the treatment of sub-acute and chronic inflammatory conditions of the respiratory tract, including COPD and Asthma. The main active ingredient of this product is LithoLexal®, a marine-derived extract containing more than 70 macro and trace biominerals.6 This plant extract retains a unique porous microstructure with a high surface-to-mass ratio, which enhances its solubility and absorption profile. More details are presented in a paper by O’Gorman et al.7 LithoLexal® as the main active ingredient in this adjunctive therapy possesses extensive anti-inflammatory effects including the suppression of IL-1β, TNF-α, and the downregulation of NF-κB.8 TNF-α and IL-1β, are known to play pivotal pathological roles in airway damage in COPD.9 LithoLexal® Respiro also provides bioactive dosages of seawater-derived magnesium (LithoLexal® MG) and vitamin D, which theoretically, can produce synergistic effects with the biominerals in LithoLexal®. Magnesium has long been suggested as a potential COPD adjunctive therapy due to its bronchodilatory effects10 whilst vitamin D possesses a diverse range of bioactivities relevant to treating the symptoms of COPD and decreasing the risk of recurrent COPD exacerbations in patients with clinical deficiency.11,12
The present study aimed to evaluate the efficacy of a 4-month intervention with LithoLexal® Respiro (Nordic Medical Ltd., London, UK) added to the baseline therapeutic regimen of adult patients with moderate-to-severe COPD. Both objective and subjective outcome measures were used to assess a more inclusive range of clinical effects.
METHODS
Study Design
This study was a single-arm, real-world study conducted in the United Kingdom (UK) to evaluate the efficacy of LithoLexal® Respiro as an adjunctive therapy in adult patients with moderate-to-severe COPD. Participants were recruited through advertisements on an online portal https://form.jotform.com/LithoLexal/litholexal-respiro—product-trial. Interested individuals applied by submitting an online form. The eligibility of each applicant was confirmed by a trained medical doctor responsible for the conduction and safety of the study. Registration for and participation in this study was voluntary, and no fee for contribution was paid to the participants.
All eligible individuals were provided with a consent form outlining the study’s purpose, the procedures involved, and their rights as participants. Volunteers were also informed that they may withdraw from the study at any time without penalty. All personal data collected during the study were kept confidential to protect the privacy of participants. The data was analyzed and reported in aggregate form to ensure that no individual participants could be identified. Provided that this research was a pilot study involving a small number of volunteers using a marketed supplement with minimal risk of side effects, no application for institutional review board approval was submitted.
Study Participants
The recruitment was conducted between 11th February 2022 and 21st March 2022 during which volunteers were enrolled from different locations in the UK. Both males and females aged 18 to 85 years with a diagnosis of COPD (confirmed by a licensed healthcare professional in the UK) who were not under systemic treatment with corticosteroids were eligible for participation.
Our main exclusion criteria were inability to use a peak flow meter (PFR), concomitant use of other COPD adjunctive therapies, recent history of cardiothoracic or other major surgeries, use of medications known to affect lung function (e.g., beta-blocking agents, immunomodulators and cytotoxic or cytostatic drugs) within the previous six months, known allergy to fish or shellfish, having the clinical manifestations of a rheumatological or other clinically significant condition. Pregnancy and breastfeeding were also considered exclusion criteria.
Study Intervention
The study intervention was two tablets of LithoLexal® Respiro (Nordic Medical Ltd., London, UK) consumed every day. Each tablet of LithoLexal® Respiro contains 1440 mg of LithoLexal® (32% elemental Ca2+ plus trace minerals) and 1212 mg of LithoLexal® MG (33% elemental MG2+). Participants were instructed to take the medication with meals and to adhere to their baseline treatment regimen throughout the study. Notably, pulmonary rehabilitation was not part of this study’s treatment protocol, and thus, none of the participants were initiated on a new rehabilitation program nor an ongoing program was stopped within the course of this study. Test products were sent to the participant’s address in 2-month batches. Receiving the next batch was conditional on a successful adherence to the study protocol. The manufacturer provided funding for the test product.
Data Collection
Our primary endpoint was the change in post-bronchodilator peak expiratory flow rate (PEFR) measured every week for four months after the start of the intervention by a standard Peak Flow Meter (PFM). This study had two secondary endpoints: 1) the change in subjective overall health of participants after being treated with LithoLexal® Respiro compared to the baseline, and 2) the safety and tolerability of the intervention.
A similar standard PFM was sent to the participants after their enrolment with clear instructions for its application. Participants measured their post-bronchodilator PEFR every week in triplicate on the same weekday in the evening with a 5-minute interval between measurements. The obtained data were recorded in a form provided by the study portal and submitted at the end of each month. Participants were also required to complete a structured questionnaire including subjective measures of clinical well-being (will be reported separately) and adherence and safety questions at the end of each month. Submitting this form was a requirement for keeping one’s position in the study.
Statistical Analysis
The primary outcome measure was analyzed using repeated-measure analysis of variance (ANOVA) to determine the effect of the intervention over time. The within-subject factor was time, with multiple measurements taken at different time points. Post-hoc pairwise comparisons were conducted using Bonferroni correction to determine the significance of differences between time points. Baseline vs endpoint overall health scores were analyzed using the Wilcoxon Signed Ranks Test to determine whether there was a significant change from baseline to endpoint. A two-tailed p-value of <0.05 was considered statistically significant for both analyses. All statistical analyses were performed using IBM SPSS Statistics for Windows (Version 22.0. IBM Corp., Armonk, NY, USA).
RESULTS
Baseline Characteristic
A total of 450 patients with the age range of 18-80 years registered for participation via the study’s online portal. Among those who expressed their interest in participation, 120 individuals were shortlisted and asked to fill in the study’s enrolment questionnaire via email. Based on the submitted information, a total of 88 eligible volunteers were enrolled in the current cohort, out of which 49 subjects finished the four phases of outcome assessments per protocol and were included in the final analyses. Figure 1 presents the number of volunteers screened, enrolled, and dropped out throughout the study.
Figure 1. Study flow diagram

Table 1 summarizes the baseline characteristics of participants. As shown, the majority of participants were 55 years or older with a long history of COPD symptoms. A noteworthy finding was the considerably low rate of treatment satisfaction among COPD patients at baseline, which highlights the need for treatment intensification.
| Table 1. Baseline Characteristics |
| Characteristics |
Number (%) |
| Age (years) |
| 25-34 |
1 (2%) |
| 35-44 |
1 (2%) |
| 45-54 |
4 (8%) |
| 55-64 |
23 (47%) |
| 65-85 |
20 (41%) |
| Sex (female) |
27 (55%) |
| Duration of COPD (years) |
| <1 |
1 (2%) |
| 1-2 |
5 (10%) |
| 2-5 |
10 (20%) |
| 5+ |
33 (67%) |
| Current Smoker |
10% |
| Satisfaction with current treatment |
| Dissatisfied |
24 (49%) |
| Relatively satisfied |
23 (47%) |
| Satisfied |
2 (4%) |
Quantitative Measurement of Lung Function
Post-bronchodilator PEFR was measured every week and reported at the end of each month through a structured online questionnaire. The highest value from triplicate measurements were recorded at each timepoint (displayed in Table 2).
| Table 2. Descriptive Statistics of Peak Flow Meter Readings from Baseline to Endpoint |
| Time (week) |
Mean (L/min) |
Standard Error |
95% Confidence Interval |
| Lower Bound |
Upper Bound |
| 1 |
229.681 |
13.441 |
202.626 |
256.736 |
| 2 |
238.830 |
14.477 |
209.689 |
267.970 |
| 3 |
243.106 |
13.978 |
214.971 |
271.242 |
| 4 |
249.000 |
15.326 |
218.150 |
279.850 |
| 5 |
252.234 |
15.048 |
221.943 |
282.525 |
| 6 |
254.255 |
15.589 |
222.876 |
285.634 |
| 7 |
255.213 |
15.611 |
223.789 |
286.636 |
| 8 |
257.660 |
15.470 |
226.521 |
288.798 |
| 9 |
260.106 |
15.447 |
229.013 |
291.200 |
| 10 |
253.511 |
14.784 |
223.752 |
283.269 |
| 11 |
257.872 |
15.254 |
227.168 |
288.576 |
| 12 |
260.745 |
15.480 |
229.585 |
291.905 |
| 13 |
261.915 |
15.677 |
230.359 |
293.470 |
| 14 |
260.851 |
14.779 |
231.103 |
290.599 |
| 15 |
260.638 |
15.386 |
229.669 |
291.608 |
| 16 |
262.766 |
15.118 |
232.335 |
293.197 |
| 17 |
265.745 |
15.242 |
235.063 |
296.426 |
As displayed in Figure 2, the mean maximal flow rate started to rise in study participants from week 1 to 9 then displayed a steady plateau until week 15. It appears from our data that receiving LithoLexal® Respiro beyond 15 weeks may contribute to a further improvement in lung function.
Figure 2. The Trend of Changes in Maximal Flow Rate (L/min) after Daily Treatment with LithoLexal® Respiro (error bars Represent SD of the Mean)
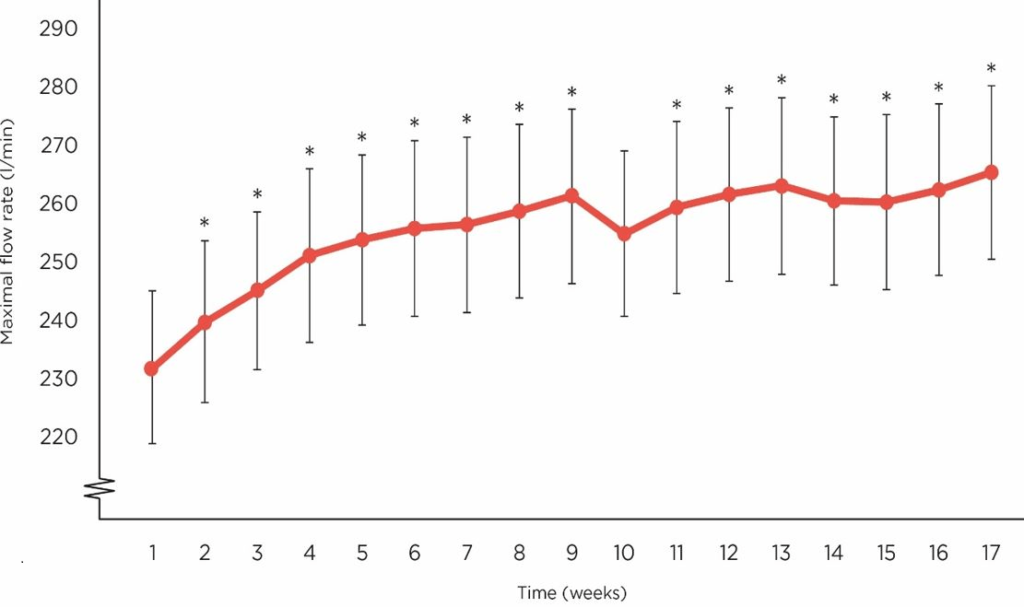
A repeated measures ANOVA was conducted to investigate the effect of LithoLexal® Respiro treatment on PEFR. Mauchly’s test of sphericity was significant (χ2 (df)=667.8 (135), p<0.001), indicating that the assumption of sphericity had been violated. Therefore, Greenhouse-Geisser correction was used to adjust the degrees of freedom.
Our analyses demonstrated that significant effects of LithoLexal® Respiro on PEFR (F (8.195, 3.70), p<0.001, ηp2=1.51), indicating that there was a significant difference in the mean PEFR values across the time course of the study. Compared to the baseline level, mean maximal flow rate increased by 15.7% at endpoint.
To determine the significance of differences between weekly measurements, post-hoc pairwise comparisons were conducted using the Bonferroni correction. The results revealed that PEFRs were significantly higher than baseline starting from week 6 (with the exception of week 10) (Table 3).
| Table 3. Pairwise Comparison of Peak Flow Meter Readings between week 1 and Subsequent Intervention Weeks; Comparisons are based on Estimated Marginal Means (Bonferroni adjusted for multiple comparisons). |
| Time a |
Time b |
Mean Difference (b-a) |
Std. Error |
p Value |
| Week 1 |
Week 2 |
9.149 |
4.249 |
1.000 |
| Week 3 |
13.426 |
3.807 |
0.131 |
| Week 4 |
19.319 |
5.646 |
0.179 |
| Week 5 |
22.553 |
5.993 |
0.064 |
| Week 6 |
24.574 |
6.199 |
0.035 |
| Week 7 |
25.532 |
6.180 |
0.020 |
| Week 8 |
27.979 |
6.535 |
0.013 |
| Week 9 |
30.426 |
7.068 |
0.012 |
| Week 10 |
23.830 |
6.351 |
0.067 |
| Week 11 |
28.191 |
6.328 |
0.007 |
| Week 12 |
31.064 |
6.543 |
0.003 |
| Week 13 |
32.234 |
7.026 |
0.005 |
| Week 14 |
31.170 |
6.695 |
0.004 |
| Week 15 |
30.957 |
6.890 |
0.006 |
| Week 16 |
33.085 |
6.829 |
0.002 |
| Week 17 |
36.064 |
7.260 |
0.001 |
Improvements in Subjective Overall Health
Study subjects were asked to rate their subjective overall health on a scale of 1 to 10 (1 being the worst imaginable health) and 10 being optimal health) at baseline and endpoint. As displayed in Figure 3, a significantly higher proportion of volunteers felt healthier after receiving the study intervention (mean (SD) score at baseline was 4.92 (1.51) and at endpoint was 6.82 (1.79)). Statistical significance of this improvement was tested using Wilcoxon Signed Ranks Test. The analysis of a total of 45 paired observations yielded a highly significant p value of < 0.001 (Z=-5.001) indicating a meaningful improvement in the perceived overall health in COPD patients after adjunctive therapy with LithoLexal® Respiro.
Figure 3. A Comparison between the Percentage of Individuals Reported Poor, Average, and Good Overall Health at Baseline versus Endpoint

Treatment Adherence and Safety
Study participants were inquired about their adherence to the study treatment at the end of each month. During the four-month study period, around 90% of subjects reported complete adherence to the treatment, while approximately 6% skipped a few dosages per month. Only one participant stopped taking the medication during the first month.
Adjunctive therapy with LithoLexal® Respiro was well-tolerated, and most participants did not report any side effects or new signs/symptoms that may potentially be associated with using the test product. Only four individuals reported symptoms, i.e., loose stool, gas/bloating, and more phlegm, that may be considered mild treatment side effects. No significant adverse effects were reported.
DISCUSSION AND CONCLUSION
The current study aimed to investigate the long term (17 weeks) effect of treatment with LithoLexal® Respiro added to the standard medical treatment of COPD on lung function and quality of life in adult individuals. The primary outcome of this study was PEFR, measured by a portable peak flow meter (PFM) every week throughout the study period. PEFR is used to assess the degree of airflow obstruction in individuals with respiratory diseases such as asthma and COPD. As a simple and non-invasive method, PEFR was shown to be efficient in detecting patients with COPD in the community and in monitoring changes in lung function over time.13
Our results revealed a steady and significant increase in mean maximal flow rate with a higher slope from week 1 to 9 and a slower rate thereafter. The mean PEFR value was increased by a clinically significant level of 36.1 L/min, (p=0.001). This observation suggests a significant therapeutic effect for LithoLexal® Respiro since the baseline treatment of patients was not changed throughout the study period. Further investigation with longer follow-up periods is needed to determine if continuing treatment beyond 17 weeks may result in higher effect sizes. We also assessed subjects’ overall health to investigate how quantitative enhancements in lung function may affect patients’ perception of wellbeing. Our findings demonstrated a positive correlation between increased PEFRs and higher-levels of subjective overall good health, which is in line with the conclusions of a multicentre cohort by Duong et al.14
To explain the pharmacological basis for the observed effects of LithoLexal® Respiro, the documented biological activities of its three main active components should be taken into consideration. LithoLexal® as the main active ingredient in this adjunctive therapy expressed a wide range of anti-inflammatory effects in controlled studies on immune cells, animal models, and humans. In a study by Ryan et al., the treatment of immune cells by LithoLexal® significantly suppressed endotoxin-induced secretion of IL-1β (by 9.5 times) and TNF-α (by 3.5 times) compared to the controls.15 Further research on macrophage lines identified that LithoLexal® has upstream anti-inflammatory effects and downregulates NF-κB by augmenting its inhibitor IkBα.8 LithoLexal® has also exhibited prominent in-vivo and clinical anti-inflammatory effects on both visceral and peripheral tissues.16,17 Supporting clinical evidence comes from a randomized, double-blind, clinical trial in which six weeks of monotherapy with LithoLexal® reduced the average serum level of TNF-α by more than 20% in patients with low-grade systemic inflammation.18 This biological activity is relevant given the fact that macrophage-derived proinflammatory cytokines, including TNF-α and IL-1β, are at the crossroads of pathologic destruction of alveolar surfaces and airway damage in COPD.9
LithoLexal® Respiro also contains a magnesium-rich compound, known as LithoLexal® MG, which is precipitated and purified from seawater. Magnesium has long been suggested as a potential COPD adjunctive therapy due to its bronchodilatory effects.19 Clinical evidence suggests that a lower-level of serum magnesium is associated with an increased risk of COPD exacerbation.20 Vitamin D is another active ingredient with a diverse range of bioactivities relevant to treating the symptoms of COPD. Vitamin D is an important moderator of immunity and inflammation owing to its integral role in the maturation and functioning of immune cells. Findings from a long-term clinical trial reported dose-dependent, protective effects for supplemental vitamin D against upper respiratory tract infection. Presumably, by reducing the rate of infections, vitamin D therapy can moderate the risk of recurrent COPD exacerbations in patients with clinical deficiency.11,12 Vitamin D also affects the function of smooth muscle cells by modulating their excitation/contraction characteristics and has an important role in the airway remodeling process.21 We hypothesize that concomitant prescription of these three active ingredients may produce synergistic effects that underlie the observed significant effect of LithoLexal® Respiro on PEFR in the current study.
Despite having a validated objective outcome measure, the current study’s limitations should be taken into account. Firstly, the study did not include a placebo control group, which could provide a more accurate comparator for assessing the true impact of the test product. A single-group design was deemed appropriate for this proof-of-concept trial to allow for a focused examination of the intervention’s impact within the study group. Secondly, the sample size was relatively small, which limits the generalizability of the findings. Practitioners are advised to exercise caution when extrapolating these findings to other populations of COPD patients. Thirdly, we used a self-administered questionnaire, home-based measurement of PEFR, and self-reported adherence, which may have affected the accuracy of the results. The study’s voluntary nature may also have introduced selection bias as individuals with greater interest or those who can afford to participate without compensation may be overrepresented.
In conclusion, the current interventional study provides initial evidence for the potential benefits of LithoLexal® Respiro treatment in improving lung function in individuals with COPD. Further research with a larger sample size, placebo control group, more accurate objective measures of lung function, and a longer duration of follow-up is warranted to confirm these findings. Nonetheless, our findings highlight the importance of treatment optimization in individuals with COPD and the potential clinical benefits of adjunctive treatments such as LithoLexal® Respiro.
INSTITUTIONAL REVIEW BOARD PERMISSION
N/A.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.









