INTRODUCTION
Evans syndrome is a disorder characterized by combined autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP). Although several previous reports were present, Evans syndrome with concurrent malignancy is rare, and cases with concurrent gastric cancer are extremely rare.1,2,3,4 The first-line treatment for AIHA and ITP is steroids, but steroid therapy to diabetic patient is almost contradictory. The second-line treatment is immunosuppressants and/or splenectomy. In recent years, efforts have been made to establish evidence supporting the use of rituximab for steroid-resistant patients.5,6 In the present case of Evans syndrome with concurrent gastric cancer, steroid therapy improved ITP, but AIHA was steroid resistant. Rituximab was effective for steroid-resistant AIHA in the present case.
CASE REPORT
The single-living patient was a 70-year-old man, 165.8 cm in height, 59.3 kg body weight and BMI 21, who had been followed-up at a local out-patient clinic for type 2 diabetes mellitus for 18 years. Obstructive pulmonary disease and post-operative cervical spine fracture were also his complaints. In December 2010, the patient felt general fatigue which deteriorated. In February 2011, he consulted a local physician for close check-up, and was found to have anemia with a hemoglobin (Hb) concentration of 7.8 g/dl. Upper gastrointestinal endoscopy revealed ulcerated lesions on the lesser curvature near the pyloric region. Biopsy of the lesion revealed funicular and sheet-like proliferative invasion of signet-ring cell-type cancer with the diagnosis of poorly differentiated stage IIc gastric cancer.
The patient refused surgery and selected follow-up observation. In March 2011, further exacerbation of fatigue was observed with a Hb concentration of 4.2 g/dL, and he was admitted to a local clinic on that day. Red blood cell (RBC) transfusion was planned to treat anemia, but all erythrocyte concentrates for transfusion were positive on pre-transfusion cross-matching test, rendering transfusion difficult. In addition, elevated lactate dehydrogenase (LDH) and positive Coombs test led to diagnosis of hemolytic anemia, and the patient was transferred to the Department of Hematology at our hospital in March 2011.
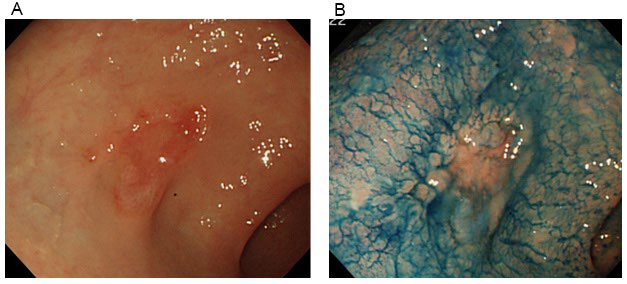
Results of laboratory tests upon admission are shown in (Table 1). Blood tests at admission revealed marked anemia and thrombocytopenia. RBC was 103 mil/m2 , Hb at 4.6 g/ dL, hematocrit at 12.8%, and platelets (Plts) at 38000 cell/μL. White blood cells at 5750 cell/μL with no abnormalities in the differential white blood cell count, reticulocytes (Ret) increased to 575%. Biochemical testing revealed increased LDH at 831 U/l, total bilirubin at 3.1 mg/dL, in which direct bilirubin at 1.1 mg/dL. In addition, direct/indirect Coombs test and antiplatelet antibody were positive, and Plt-associated IgG level was high. Bone marrow examination revealed a nuclear cell count of 70.4×104 /μL and increased erythroblasts (Figure 1). The megakaryocyte count was 104 cell/μl. There were no atypical cells, suggesting malignancy. Evans syndrome was diagnosed based upon the presence of concurrent AIHA and idiopathic thrombocytopenic purpura (ITP). In addition, re-evaluation of gastric cancer was performed using a gastrocamera (OLYMPUS model Q260) and confirmed the ulcerated lesions on the lesser curvature near the pyloric region and the incisura angularis of the greater curvature, and the pathological diagnosis was similar to the previous one (Figure 2). Computed tomography performed to deny potential gastric cancer metastasis revealed no metastatic lesions.
Table 1: Laboratory data on admission.
| WBC 5750 cell/μL (4000-8000)
stab 9%
seg 32%
lympo 37%
mono 12%
eosino 1%
baso 1%
myelo 6%
metamyelo 2%
eryblast 64%
RBC 100m/m2 (420-540)
Hb 4.6 g/dL (12.5-17.5)
Ht 13.1% (39-51)
Plt 38000/μl(130000-330000)
Ret 565 ‰ (7-23)
PT-INR 1.10 (0.9-1.13)
APTT 26.9 sec (28-40)
FIB 244.2 mg/dL (150-340)
FDP 16.3 μg/mL (0-8)
AT-3 73.2% (80-120) |
T-Bil 3.1 mg/dL (0.2-1.2)
AST 41 U/L ( 7-40)
ALT 11 U/L (0-35)
ALP 204 U/L (80-300)
LDH 831 U/L(100-225)
GLU 171 mg/dL (65-110)
BUN 15.3 mg/dL (8-20)
CRN 0.8 mg/dL (0.7-1.3)
UA 7.9 mg/dL (3.8-8)
TP 7.0 g/dL (6.5-8.2)
ALB 3.3 g/dL (3.7-5.5)
CRP 3.74 mg/dL (0-0.3)
Na 136 mEq/l (135-145)
K 3.8 mEq/l (3.4-4.9)
Cl 107 mEq/l (98-108)
Fe 209 μg/dl (80-200)
UIBC 46 μg/dl (90-275)
Ferritin 282 ng/ml (21-282)
VitB12 9675pg/ml (233-914)
Folic acid 1.2ng/ml (3.6-12.9)
CEA 4.9 ng/ml (0-5)
CA19-9 2.2 U/ml (0-37) |
IgG 1997 mg/dL (1000-1900)
IgA 358 mg/dL (96-430)
IgM 48 mg/dL (48-350)
HPT 2 (19-170)
DCT/ICT+
CA <4
PaIgG 22 ng/10 cells(0-25)
Antiplatelet Ab +-(negative)
RA 3 IU/ml (0-15)
ANA –
Anti-DNA Ab <80
BM
NCC 70.4×104 cell/μl
MgK 104 cell/μL
No malignancy cell
Karyotype
46,XY |
Figure 1: Images A and B of bone marrow examination.
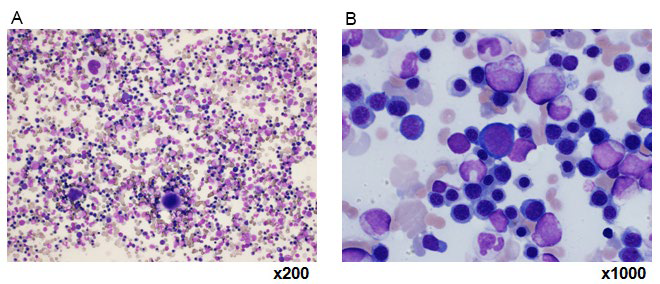
Figure 2: Endoscopic findings of the stomach.
PROGRESS ON ADMISSION
As summarized in Figure 3, prednisolone (PSL) was administered at a dose of 60 mg/day (1 mg/kg/day) to treat Evans syndrome (Figure 3). Although exacerbation of hemolysis was afraid due to blood typing and cross-matching test, and a compatible blood transfusion was challenging, a total of six units of RBC were transfused, and no clear deterioration of hemolytic findings happened following transfusion. Anemia persisted, however, and signs of cardiac failure developed.
Figure 3: Clinical course.
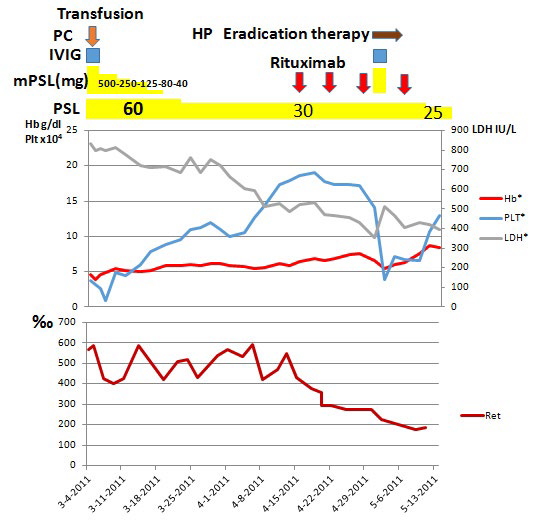
On day 3 of hospitalization, the patient’s Platelet count markedly decreased to 9000 cell/μL. Considering the risk of hemorrhage from the gastric cancer, pulse therapy with methylprednisolone (mPSL) was administered on the same day at a dose of 500 mg/day for 3 days. In addition, with 20 units of platelet transfusion and gamma globulin at 5 g/day for 3 days were added to enhance the effect of transfusion. One hour later, the platelet transfusion count increased to 64000 cell/μL and was gradually restored; however, no improvement in anemia was observed. Although insulin had been used for steroid treatment at admission, blood glucose became 545 mg/dL with poor control, so the mPSL dose was gradually decreased after the pulse, and PSL at a dose of 30 mg/day (0.5 mg/kg/day) was continued for an additional 4 weeks.
However, his anemia did not improve, nor did lower Ret and LDH levels. Therefore, the diagnosis of steroid-resistant AIHA was done. As the Plt count improved, surgical resection of the gastric cancer and splenectomy were planned to avoid the risk of intraoperative hemorrhage and to delete the cancer-induced autoantibody to yield paraneoplastic syndrome. However, the patient strongly refused the surgical resection, because one of his relatives had died under the surgery for gastric cancer.
Severe anemia persisted and emergence of cardiac failure deemed it imperative to expeditiously improve the patient’s anemia, so rituximab administration was considered. After receiving consent from the patient and his family, rituximab was administered on day 42, total of four times at a dose of 375 mg/m2 once a week. Following rituximab therapy, LDH and Ret count decreased, and anemia gradually improved. As a decrease in Plt count caused by a decrease of steroids was observed, on day 62, repeat mPSL at 500 mg/day and gamma globulin therapy at 5 g/day were administered for 3 days when the Platelet count was 39,000 cell/μL. The patient was positive for Helicobacter pylori, for which pylori eradication therapy was initiated. Thereafter, the anemia abated, the Platelet count was restored, and the patient discharged. Although the patient’s gastric cancer was eligible for surgical treatment, the patient refused any further treatment.
Thereafter, eating disturbances appeared due to the progression of gastric cancer, and the patient underwent surgery at a different hospital. We were notified that there was no recurrence of AIHA or ITP.
DISCUSSION
Evans syndrome is a merger of AIHA and ITP. Both are autoimmune disease, so that the first treatment is a steroid. Treatment guidelines for AIHA recommend steroids at a dose of 1 mg/kg as the first-line therapy. Elderly patients require more scrutiny for potential complications including diabetes and infection. The Patient had diabetes, but early steroid treatment was required for heart failure and bleeding tendency. As he had gastric cancer, microangiopathic hemolytic anemia should be ruled out.7
Approximately 40% of patients with AIHA achieve remission in approximately 4 weeks with steroid therapy. In the event of ineffective first-line therapy with steroids, a secondline therapy is initiated; these include, in the absence of an underlying disease, splenectomy or immunosuppressive therapy individually determined based on each patient’s condition.
Rituximab, anti-CD20 antibody, selectively targets B lymphocytes and reduces autoantibody production. Recently, numerous reports of cases in which rituximab was used for refractory AIHA have examined its efficacy and safety. Penalver et al5 treated patients with refractory AIHA with rituximab administered once a week at 375 mg/m2 for a total of 4 doses and achieved a 77% response rate. Furthermore, Barcellini et al6 achieved a response rate of 82% with rituximab at a low dose of 100 administered 4 times. Thus, rapid and continuous therapeutic outcomes with rituximab should be anticipated. In patients with an underlying disease such as diabetes who are unsuitable for long-term steroid treatment, rituximab is suggested to be a more effective approach.
Secondary AIHA may develop in the setting of an underlying disease such as connective tissue disorders, particularly systemic lupus erythematosus, lymph proliferative diseases, malignant tumors, certain medications, and infection. The mechanism is suggested to involve alterations in patient RBC antigens resulting in their cross-reactivity with antibodies against foreign antigens and changes in the immune system.
Gastric cancer concurrent with AIHA is rarely observed. Furthermore, only a few reports describe Evans syndrome including both AIHA and ITP.1 In 2010, a meta-analysis by Joe et al2 evaluated 52 reported cases of solid cancer concurrent with AIHA between 1945 and 2009. In their report, the most common cancers were pulmonary, renal, and colorectal cancer. There were only a few reports of Evans syndrome with gastric tumor.4,8 The analysis showed that patients who responded to steroid therapy as first-line treatment subsequently underwent surgical resection. In addition, a subset of patients with steroid-refractory AIHA has been reported to subsequently improve by surgical resection. Furthermore, our literature search revealed only 3 reports of gastric tumor concurrent with Evans syndrome; the types included neuroendocrine tumor, plasmacytoma, and signet ring cell type gastric cancer. Signet ring cell cancer is relatively rare in the stomach, so the association with AIHA is noteworthy
Surgical resection of gastric cancer would have enabled us to determine whether Evans syndrome was paraneoplastic syndrome of the tumor. However, because the patient refused surgery, it could not be confirmed. The patient received gastrectomy after discharge, and the Evans syndrome has not been recurrent, a possibility of paraneoplastic syndrome could be accepted.
Rituximab therapy, which was initiated as an alternative, resulted in rapid decrease of Ret count and LDH, and early therapeutic effects with only a mild decrease in IgG were observed. Rituximab administration was safe without any major side effects. Currently, although there is insufficient supporting evidence for rituximab therapy in AIHA,5,9,10,11 we believe that rituximab is a beneficial therapy for steroid-resistant AIHA, with a favorable safety and efficacy profile.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










