INTRODUCTION
Chronic Myelogenous Leukemia (CML) is a myeloproliferative disorder characterized by the existence of Philadelphia chromosome. BCR-ABL protein targeted drugs called tyrosine kinase inhibitors (TKIs) dramatically improved patients’ survival in 2010s. These drugs are imatinib, Nilotinib, Dasatinib and Radotinib.
As a result, hematopoietic stem cell transplantation (HSCT) with allo-HSCT is decreasing.1 A graft failure is one of the crucial complications in allo-HSCT. If it occurs, the most of the patients run their fatal course. In the present case, promyelocytic blast crisis occurred in the second chronic phase CML and we performed allo-HSCT for three times for graft failures.
CASE REPORT
A 42 year-old male was diagnosed with CML in June 2011 and started the administration of nilotinib of 600 mg/day on June 12, 2011. He was treated by TKI, and a month later, therapeutic effect reached complete hematologic response.Then, 5 months later, it reached complete cytogenetic response although it had never reached major molecular response ever.
We considered changing nilotinib to dasatinib at the 19th month since it did not reach major molecular response at the 18th month.2 On January 20, 2013, laboratory data showed pancytopenia; white blood cell count of 1.76×109 /L, hemoglobin count of 14.6 g/dL and platelet count of 122.0×109 /L. Biochemical testing showed asparatate transaminase (AST) at 33 U/L, alanine aminotransferase (ALT) at 218 U/L, lactate dehydrogenase (LDH) at 148 U/L, total bilirubin (T-Bil) at 2.3 mg/dL and CReactive Protein (CRP) at 0.05 mg/dL. Coagulation test showed prothrombin time (PT)-INR at 0.96, activated partial thromboplastin time (APTT) at 27.4 sec, fibrinogen (Fib) at 212 mg/dL, fibrin/fibrinogen degradation product (FDP) 1.6 µg/mL. A smear of bone marrow aspiration showed promyelocytic blastoid cells 31.4% with positive cluster of differentiation 16 (CD16), CD33 and human leukocyte antigen-antigen D related (HLA-DR) staining (Figure 1A and B). RT-PCR showed promyelocytic leukemia gene/retinoic acid receptor alpha (PML/RARα) 47000 copy/µRNA on admission (Table 1). Thus, promyelocytic (M3) blast crisis of CML was diagnosed. Moreover, BCR/ABL was 625 copy/0.5 µRNA and there was no gene mutation of BCR/ ABL. Karyotype revealed 46, XY, t(9;22)(q34;q11.2), t(15;17) (q22;q21)9/20 by Giemsa band (G-band) staining and Spectral karyotyping-Fluorescence in situ hybridization (SKY-FISH) (Figure 2).
Figure 1: Image of Bone Marrow Examination
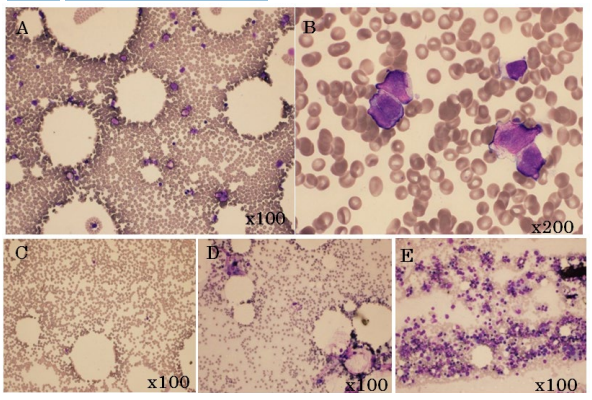
The Figure A,C,D and E is x200. The Figure B is x1000. The Figure of A and B Show Promyelocytic Blastoid. The Figure of C and D Show Hypocellular Bone Marrow as Graft Failure. The Figure of E Show the Engraftment.
Table 1: Hematological Data at the First Admission, Blastic Crisis and Discharge.
| |
At first |
At blast crisis |
At discharge |
|
WBC (4000-8000) /μL
|
25300
|
1510
|
2480
|
|
stab %
|
2.5
|
1
|
1
|
|
seg %
|
59.5
|
41
|
40
|
|
lymp %
|
17.5
|
55
|
41
|
|
Mono %
|
6.5
|
2
|
13
|
|
Eosino %
|
1.5
|
0
|
4
|
|
Baso %
|
3.5
|
1
|
1
|
|
Myelo %
|
7.5
|
|
|
|
Metamy %
|
1.5
|
|
|
|
RBC (420-540) m/mm2
|
543
|
416
|
277
|
|
Hb (12.5-17.5) g/dL
|
16.6
|
13.6
|
9.9
|
|
Ht (39-51) %
|
50.3
|
40.1
|
29.5
|
|
Plt (130000-330000) /μL
|
284000
|
136000
|
147000
|
|
Ret (7-23)
|
22.4
|
13.6
|
35.6
|
|
BM
|
|
|
|
|
NCC /μL
|
1117000
|
84000
|
*105000
|
|
MgK /μL
|
343
|
12
|
*50
|
|
Promyelocyto(Blastiod) %
|
|
31
|
*0.6
|
|
M-bcr/abl mRNA copies/ μgRNA
|
27000
|
180000
|
*<50
|
|
PML/RARα mRNA copies/ μgRNA
|
|
47000
|
*<50
|
In parentheses indicates the normal value.
*is the data of two months before discharge.
Figure 2: karyotype Test. A Chromosomal Abnormality by G-Banding and SKY FISH.
Red Circle is an Abnormal Place.

It was positive of BCR/ABL and PML/RARα; therefore, the administration was changed from nirotinib to dasatinib in addition to administer ATRA on January 25, 2013. On March 19, 2013, bone marrow examination revealed to reach complete hematologic remission with negative of BCR/ABL and PML/ RARα by fluorescence in situ hybridization, but they were positive by the reverse transcription-polymerase chain reaction (RTPCR).
Hence, this therapeutic effect reached complete cytogenetic response (CCyR) but did not reach major molecular response (MMR). Thus, we diagnosed that his state could achieve second chronic phase. According to the course of treatment until then, we had been considering that it was necessary for him to be performed allogenic hematopoietic stem cell transplantation. Therefore, we began to search for donors immediately.
He had a human leukocyte antigen (HLA) well matched related donor, a sister; however, she had past medical history of hepatitis C and had undergone medical treatment for Graves’ disease by methimazole prior to the eligibility test. Based on these factors, we decided she did not suit as his donor, so that we started searching for an unrelated donor. Finally, we selected cord blood for his treatment since there was no well-matched unrelated bone marrow donor in Japan Marrow Donor Program.
Selected cord blood was from a female, its donor-recipient HLA-allele match was 5/8, nuclear cell count was 3.71×106 /kg, CD34 positive cell count was 0.756×105 /kg and blood type was A+ (recipient blood type was B+). HLA antibody was positive yet donor allele type’s antibody did not exist. On May 9, 2013, he was hospitalized for allogenic hematopoietic stem cell transplantation.
Stem Cell Transplantation
The conditioning regimen was selected as myeloablative regimen, intravenous busulfan and cyclophosphamide (IV BU/CY) and prevention of graft vs. host disease, was used for cyclosporine and short-term methotrexate. Transplantation conditioning was started from 7 day and cord blood (CB) infusion was performed on 0 day (Figure 3). It caused myelosuppression and caused a grade 2 fever and a grade 3 sore throat. Rush appeared on the 20% of body surface area mainly on the trunk from day 15 and his white blood cell (WBC) count decreased to 0.18×109 /L on 18th day. Therefore, we diagnosed that it was an engraftment syndrome by a febrile neutropenia.
Figure 3: The Figure Explains the Course of Three Bone Marrow Transplantation. Purple, Blue and Green Line Shows WBC, Neutrophil and Platelet. Red and Yellow Line Show Body Temperature and CRP. We Checked The Engraftment by FISH for Heterosexuality and Microchimerism for Same Sex at Black Arrow.
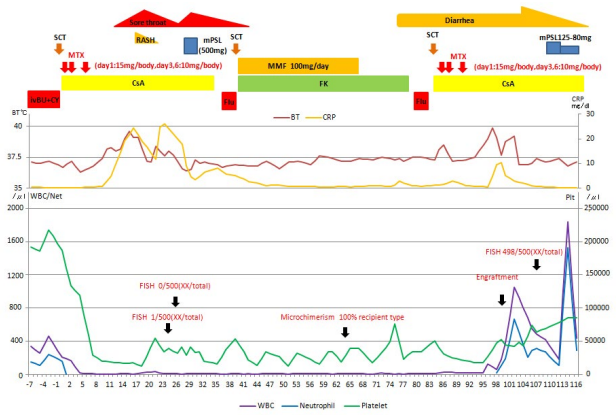
According to his good physical status and no abnormal vital sign, observation on the engraftment syndrome was continued by administration of antimicrobial drug for febrile neutropenia. On the day 21, his fever improved and white blood count became 0.31×109 /L and the value of C-reactive proteinimproved. However, fever and sore throat was getting worse and a severe neutropenia happened on the day 23 and 25 in which WBC count decreased to 0.09×109 /L. A possibility of hemophagocytic syndrome (HPS) was suspected, and bone marrow examination revealed sever hypocellular bone marrow; nuclear cell count was 3.0×109 /L without hemophagocytic macrophage (Figure 1C). Ferritin level on the day 25 and 28 were 1190.7 ng/ mL and 974.2 ng/mL, respectively. AST, ALT and other hepatic enzymes were within normal levels and no splenomegaly was noticed. Thus, the hemophagocytic lymphohistiocytosis-2004 (HLH) trial criteria were not satisfied.
These conditions seemed to be the secondary graft failure by HPS, so 500 mg methylprednisolone was given for 3 days from the day 28. On the day 30 the severe hypo-cellular bone marrow showed chimaerism of complete recipient pattern.
He was diagnosed with secondary graft failure and the second HSCT was performed. We selected CB from male, and blood type A+. Whose recipient-donor HLA allele match was at 4/8, nuclear cell count was 2.13×106 /kg, and CD34 positive cell count was 0.495×105 /kg. Donor allele type’s HLA-antibody had not been revealed prior to the second HSCT. We selected non-myeloablative conditioning of fludarabine 30 mg/m2 for 3 days, because he had an infection as a complication of HSCT. Fujisawa kaihatsu/mycophenolate mofetil (FK/MMF) (MMF 1000 mg/day) was selected for prevention of graft versus host disease (GVHD) on the 40th day from the first HSC infusion.
After the second HSCT, his fever and sore throat improved but white blood cell count remained low. Bone marrow examination performed on the day 65 after the second SCT showed a severe hypo-cellular bone marrow in which nuclear cell count was 5000/µL (Figure 1D) and chimaerism was exactly the same as the pattern of the recipient before.
Therefore, we decided that the second HSCT was failed.
On the third HSCT, there was no CB better than the second SCT. Thus, we extended the range of donors to cousins to consider a better possibility of haplo-identical hematopoietic stem cell transplantation. However, there were no haplo-identical donors. We thought there was no superior method to save him besides the transplantation from his sister mentioned earlier who was HLA well matched related donor. We had got sufficient informed consent from him, his family and donor candidate, and she agreed to be a donor.
Her aptitude for SCT was confirmed by hepatitis C virus (HCV) infection being below the sensitivity level by qRTPCR, and the thyroid function was within the normal range. Third HSCT was decided to perform peripheral blood hematopoietic stem cell transplantation, because the recipient had been in continued long-term neutropenia. Conditioning regimen was the same as the second SCT, and prevention of GVHD were done by CyA/ shot-term methotrexate (MTX) (day1:15 mg/body day 3 and 6:10 mg/body). Peripheral blood stem cell harvest was performed to use granulocyte-colony stimulating factor (GCSF) 10 µg/kg/day. Collected nuclear cell counts (NCCs) was 7.26×108 /kg and CD34+ cells 2.39×106 /kg. Stem cell transplantation (SCT) was performed on the day 84 and 85 after operating the 1st SCT. White blood cell count increased from the day 11 and became over 1000/ml on the day 99. He had been having a fever, decreasing percutaneous oxygen saturation and increasing body weight due to edema from the day 98. Those symptoms apparently started progressive from the day 102. Acute GVHD or engraftment syndrome was suspected and 125 mg methylprednisolone (mPSL) was administrated. Diarrhea, fever and respiratory distress were improved after administration of mPSL. Colonoscope was performed for the diagnosis of GVHD, but no pathological finding of GVHD was found. We considered these symptoms were related to the graft failure. Therefore, we decided to decrease the mPSL administration. Bone marrow examination on the day 107 for confirming graft engraftment showed enough hematopoiesis (NCC:73.1×104 /µL) (Figure 1E). The graft engraftment by in situ hybridization was donor type 498/500. Engraftment syndrome was improved so that administration of mPSL was discontinued and CyA was changed intravenous administration to per oral administration (PO) on the day 113. Stage acute GVHD appeared on the skin on the day 155; however, this symptom was successfully controlled by antihistamine and external preparation of steroid.
CyA induced renal dysfunction was improved by decreasing CyA and discontinue on the day 193. PSL 10 mg administration was started from the day 114 because a GVHD still remained. Twelve months later from the third SCT, CML condition retained MMR, HCV level kept below the sensitivity level, thyroid function was normal and thyroid stimulating hormone (TSH) receptor antigen was negative, and he could discharge from the hospital.
DISCUSSION
Graft failure is one of the rare yet crucial complications of alloSCT. Fatality rate is said to be 40-50% when it occurs. Basically, radical treatment of CML is the only allo-SCT except it caused HPS.3,12Therefore, second allo-SCT should be performed as soon as possible after the diagnosis of graft failure. So far, our searched on previous cases which had cured from two times graft failure, found no report of multiple SCT with successful result. Cell number of graft, basic disease, disease
stage before SCT, degree of HLA matching, blood type mismatch, cord blood, existing of HLA antibody, prevention of GVHD and conditioning regimen were considered to be the cause of graft failure. Especially, cord blood was known to cause high rate of graft failure, it is recognized at approximately 20% of the cases. Cord blood, HLA antibody, blood type mismatch, conditioning regimen and degree of HLA matching corresponded to our case.
There was no large cohort study describing therapeutic value of cord blood SCT for CML. Currently, there is one retrospective report of 86 cases from Japan Cord Blood bank.5 The report showed that 2 year predicting event-free-survival (EFS) was 34±6%, leukemia-free-survival (LFS) was 38±6% and overall-survival (OS) was 53±6% after cord blood stem cell transplantation for CML. In addition, with two-year EFS for patients in chronic phase (CP), accelerated phase (AP) and blastic crisis (BC) was 52% (95% CI, 56-90%), 38% (95% CI, 17-84%) and 22% (95% CI, 10-48%) and with two-year OS for patients in CP, AP and BC was 71% (95% CI, 48-100%), 59% (95% CI, 37-94%) and 32% (95% CI, 20-55%), respectively. Cord blood could be acceptable as alternative donor for CML patients who need allo-SCT when well-matched related or unrelated donor was present. Otherwise, this report said that EFS makes a significant difference in nuclear cell count (NCC) and the age of the recipient. Event-free survival (EFS) of the case of NCC above 3.0×107 /kg was 68% (95% CI, 48-96%) and below it was 20% (95% CI, 12-35%.). Its p value was at 0.0005. EFS significantly differed by age; under the age 15 was 74% (95% CI, 48-100%), between the age of 15 and 50 was 33% (95% CI, 22-49%) and overthe age of 50 was 15% (95% CI, 3-72%).
There were 7 cases of graft failure in the report, but the relationship with NCC and age was not mentioned. In this case, age of our patient had a capability of a risk factor, although cord blood had enough NCC at the first SCT. Nowadays CD34+ cell count showed a better relationship than NCC for graft engraftment. Takahashi et al reported that HLA antibody is related to WBC engraftment but the cases are not effected by that in case cord blood having 0.85×105/kg CD34+ cells or more.1,2,3,4The first and the second SCT had 0.445×105/kg and 0.5×105/kg CD34+ cells so CD34+ cells count was suspected to be the reason of graft failure (Table 2). From the above factors, it is necessary for us to consider CD34+ cells count in case recipient who has HLA antibody.
| |
1st
|
2nd
|
3rd
|
|
Donor source
|
Cord Blood |
Cord Blood |
Peripheral blood ( sibling) |
|
HLA match
|
5/8 |
4/8 |
8/8 |
|
Blood type
|
Major minor mismatch |
Match |
Match |
|
NCC
|
3.71×106 /Kg |
2.13×106 /Kg |
7.26×107 /Kg |
|
CD34+ cell count
|
0.756×106 /Kg |
0.495×106 /Kg |
2.39×106 /Kg |
|
Conditioning regime
|
ivBU(12.8 mg/kg)×4/ CY(120 /m2 )x2 |
Flu( 30 mg/m2 )X3 |
Flu( 30 mg/m2 )X3 |
|
GVHD prevention
|
CsA+Short term MTX |
Tac+ MMF |
CsA+Short term MTX |
|
Result
|
Graft failure |
Graft Failure |
Engraftment |
In recent years, cases of M3 type blast crisis of CML have been reported over 30 cases and 7 cases were treated by TKIs. Some cases showed the effect of chemotherapy, combine all-trans retinoic acid (ATRA) or arsenic trioxide. On the other hand, allo-SCT were performed in 3 cases and these cases showed therapeutic effect ranging from CCyR to MMR. AlloSCT is considered to be an effective therapy for these cases.
In comparison of myeloablative conditioning regimen, formerly, busulfan and cyclophosphamide (BU-CY) had a weaker immune suppression than cyclophosphamide-total body irradiation (CY-TBI). Employment of BU-CY leads graft failure more frequently in both peroral BU-CY and CY-TBI to CML patients.6 A retrospective cohort study showed intravenous BUCY was superior to CY-TBI on OS of CML patients.7,8 Based on these reports, conditioning regimen of first CBT was not suspected to be a factor of graft failure.1,2,3 If the blood concentration of BU is below its effective concentration, frequency of graft failure would increase. Area under the blood concentration-time curve (AUC) of intravenous BU administration is more stable than preoral BU. Almost all cases fit in effective concentration 800-1500 µM-min though approximately 14% of the cases do not fit in.9,10 There is a possibility that AUC is below the effective concentration because our case did not measure BU AUC.
We had no consensus on the conditioning regimen of re-transplantation for graft failure yet. There is a tendency that fludarabine based nonmyeloablative regimen is selected more. Our case was chosen 3 day fludarabine regimen. Recently, the one-day regimen which is combined fludarabine with
alkylating agents, anti-human thymocyte immunoglobulin (ATG) or alemtuzumab has been reported and some cases could have beentreated by this regimen.4,11
Second CBT was considered to have an extremely high risk for graft failure since it was cord blood for the second time and its cell count was below 3.0×107 /kg. Therefore, we had to select a donor as soon as possible. The HLA well-matched relative donor had been infected of hepatitis C and on medication for Grave’s disease prior to the transplant, so we had selected her as an urgent donor. As leukocytopenia and neutropenia had continued long term, the patient understood the necessity of the third allo-SCT well. He was enthusiastic for infection prevention so that we could perform the third allo-SCT. Now, he is able to return to his occupation without any infection with hepatitis C. Moreover, his thyroid function is normal. It would be necessary for the patient to be carefully observed for further hepatitis and thyroid function.
ACKNOWLEDGMENT
We would like to thank Dr. Shaw Watanabe for good his constructive advice.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








