INTRODUCTION
Mycotoxins are environmental pollutants present in virtually all parts of the world. More than 300 chemically different mycotoxins formed by more than 350 fungal species and causing diseases (mycotoxicoses) to living organisms are described.1 As yet only a few mycotoxins have been related to important food- and feed-borne diseases, the potential impact on human and animal health of many of them remains to be elucidated. The most frequently toxinogenic fungal species found in food and feed commodities belong to the genera Aspergillus, Fusarium and Penicillium.
Reproductive failure and a drop in reproductive performances are brought on by mycotoxins. Zearalenone produces in farm animals a true estrus enlargement of both vulva and uterus and other general responses associated with estrogens.2,3 Zearalenone has strong estrogenic effects, as well as haematotoxic and genotoxic properties.4 Higher doses of zearalenone affect ovulation, conception, implantation, foetus development, and the newborn’s viability. Prepubertal female pigs are the most affected farm animals by zearalenone. Zearalenone produced hyperestrogenism in young gilts and delayed cycling in prepuberal gilts.5 Ochratoxin A causes a remarkable delay in sexual maturation in rats on account of suppressed ovarian steroidogenesis.6,7
Long-term exposure to low concentrations of zearalenone leads to impairment of proliferative activity of the follicle granulosa cells and connective tissues of the ovarian stroma in prepubertal gilts.8 In gilts, zearalenone caused follicle atresia and apoptotic changes in granulosa cells.9 In pregnant and lactating sows exposed to the action of zearalenone, the number of follicles with normal morphology was decreased. This reduction of reserves of the early stages of follicles can cause the premature depletion of healthy follicles and shorten the reproductive period in sows.10 The derivatives of zearalenone, α- and β-zearalenol, inhibit progesterone synthesis in porcine granulosa cells.11
Many mycotoxins have direct effects on the fetus12 and have teratogenic effects.13 For the investigation of antifertility activity, various experimental parameters were reported earlier e.g., delay in sexual maturity,14,15 resorption of fetuses in pregnant rats,16 direct effects on fetuses.17 At present it is supposed that among many other factors, gonadal steroids have an important role in the maintenance of oestrus cycle and thus ovulation.
The mycotoxin problem has attained considerable significance in terms of public health and animal husbandry. It is therefore a matter of any national interest to make appropriate studies to deal with the problem of isolation, identification and toxicological evaluation of mycotoxins as contaminants in human diet and their consequent effects on animals. From this standpoints, mycotoxin MT81 was isolated, purified and identified in our laboratory from a locally isolated fungal strain of Penicillium nigricans, (patent no. 156916 dated 15.2.82, Government of India). MT81 is a dextrorotatory polyhydroxyanthraquinone compound (molecular formula, C22H18O7 and Molecular weight, 394) and its LD50 value is 35.1 mg/kg body weight in mice. MT81 is a good antimicrobial,18 hyperglycemic,19 and antileishmanial20 agent. It produces liver,21 brain22 and kidney dysfunction23 and massive bone marrow depression.24
Besides their toxicological effects, mycotoxins may possess good antibiotic activities. From this aspect, some mycotoxins can be used as medicine if their toxicity can be reduced. So, Benzoylated derivative of MT81 (Bz-MT81) was synthesized in our laboratory to generate a more potent and less toxic compound (LD50 values 87.1 mg/kg body weight in mice) in order to find out good therapeutic agent which may lead to more effective drug formulation. This derivative possesses antibacterial, antifungal18 and antileishmanial20 effects.
India is a country where population density is significantly higher. So this present study was an attempt to synthesize a less toxic compound and to search out its efficacy as an antifertility agent. At the same time, the present study was designed to investigate the comparative antifertility activities of mycotoxin MT81 and its benzoylated derivative on the reproductive system of prepubertal female albino Wistar rats by assessing the ovarian and adrenal activity as well as steroidogenesis in these organs.
MATERIALS AND METHODS
Chemicals and Reagents
MT81, Bz-MT81 were synthesized in our laboratory. Nicotinamide adenine dinucleotide, Dehydroepiandrosterone sulphate, Nicotinamide adenine dinucleotide phosphate, Glucose-6-phosphate, Bovine serum albumin were purchased from Sigma Aldrich Inc., USA. All other chemicals used were purchased from Himedia India Ltd. Merck India Ltd. etc.
Animals
Ninety closed colony, randomly breed albino Wistar prepubertal female rats were taken at 25 to 30 days of age (weighing 50 to 60 g). The rats were housed in cages under standard conditions (12:00 h light: 12:00 h dark, 25±2 °C) with a standard laboratory pellet food and drinking water ad libitium. The animals were acclimatized for 15 days before experimentation. The study was approved by the Institution’s animal ethical committee.
Treatments
Ninety albino Wistar prepubertal female rats were equally divided into six groups, each group comprising of 15 rats. Treatments were done intraperitoneally with MT81 and benzoylated MT81 dissolved in propylene glycol and it was carried out on every alternate day for 14 days (total 7 doses). Alternate dosing is comparatively safe and tolerable for the treated animals as the LD50 value of MT81 is low. We selected the dose and experimental schedule according to our previous study.25
The groups and treatments were as follows:
Group-I : Saline control (0.1 ml of 0.9 % NaCl/ 20 g of body weight)
Group-II : Vehicle control (0.1 ml of propylene glycol/ 20 g of body weight)
Group-III : 5 mg /kg (0.1 ml MT81/ 20 g of body weight)
Group-IV : 7 mg/kg (0.1 ml of MT81/ 20 g of body weight)
Group -V : 7 mg/kg (0.1 ml of Bz-MT81/ 20 g of body weight)
Group-VI : 9 mg/kg (0.1 ml of Bz-MT81/ 20 g of body weight)
Total body weights of the rats in each group were taken before and after the treatment period. Sixty rats were sacrificed 24 hours after the last dose and food was withdrawn 18 hours before sacrifice though drinking water was supplied sufficiently. Five rats of each group were kept for the study of the age of vaginal opening and appearance of the first estrous.
Biochemical Assays
Ovaries, uterus and adrenal glands were dissected out from each animal immediately after sacrifice and were done free from fat and the weights were recorded. The ovaries and adrenal glands were taken out for the estimation of cholesterol,26 ascorbic acid,25 glucose-6-phosphate dehydrogenase,27 ∆5-3-beta-hydroxyesteroid dehydrogenase.25
Estimation of Ovarian and Adrenal Cholesterol
Ovary and adrenal gland of each rat were homogenized in 0.25 sucrose solution for the estimation of cholesterol. 4.75 ml of ethanol and acetone mixture was added to 0.05 ml of homogenate (2%, w/v). The mixture was shaken well and kept for 10 min. The mixture was then centrifuged at 3000 rpm for 10 min. 1 ml of the supernatant was evaporated to dry in boiling water bath, then the residue was mixed with 3 ml of glacial acetic acid and warmed for 20 min. After addition of 2 ml color reagent (10% FeCl3, 6 H2O, 100 ml acetic acid, conc. H2SO4), the reading were taken at 560 nm within 10 min. The amount of cholesterol present is calculated by plotting the standard curve.26
Measurement of Ovarian and Adrenal Ascorbic Acid
For the estimation of ascorbic acid, the ovarian and adrenal tissues were homogenized using 2.5 ml of 5% metaphosphoric acid-10% acetic acid solutions. The mixture was centrifuged after extraction and a very small drop of concentrated bromine was added to the supernatant. Tube was shaken and kept for 10 min for complete oxidation. Excess liquid bromine was then removed. 0.5 ml of dinitrophenylhydrazine–thiourea reagent (2.2% 2, 4-DNPH in 100 of 10N H2SO4, 5% thiourea) was added with 2 ml of tissue extract and incubated at 37 °C for 3 h and then 2.5 ml of 85% H2SO4 was slowly added in ice-cool condition. It was mixed well for half an hour in room temperature for color development and optical density was observed at 540 nm.25
Assay of Ovarian and Adrenal Δ5-3β-Hydroxysteroid Dehydrogenase Activity
For the estimation of ovarian and adrenal ∆5-3β-hydroxysteroid dehydrogenase (HSD) activities28,29 one ovary and adrenal gland from each animal were taken. The tissues were homogenized carefully at 4 °C in 20% glycerol containing 5 mM potassium phosphate and 1 mM EDTA to make a tissue concentration of 100 mg/ ml homogenizing mixture. Then the mixture was centrifuged at 10,000 g for 30 min at 4 °C. The supernatant (1 ml) was mixed with 100 µm sodium pyrophosphate buffer (pH 8.9), 40 µl of ethanol containing 30 µg dehydroepiandrosterone and 960 µl of 25 mg% Bovine Serum Albumin (BSA), to make the incubation mixture to a total of 3 ml. In spectrophotometer cuvette, after addition of 0.5 µM NAD to the tissue supernatant mixture, enzyme activity was measured at 340 nm against a blank (without NAD). One unit of enzyme activity was the amount causing a change in absorbance of 0.001 per minute at 340 nm.
Assay of Ovarian and Adrenal Glucose-6-Phosphate Dehydrogenase Activity
Glucose-6-phosphate dehydrogenase (G-6-P-D) activity was assayed biochemically by monitoring the formation of NADPH at 340 nm.27 The tissue was homogenized with 0.5 ml of Tris buffer (pH 7.4). Then it was centrifuged at 7000 rpm for 15 min at -20 °C. In a cuvette, 0.02 ml of extract, 0.2 ml of 0.5 M Tris-HCl buffer (pH 8.3), 0.01 ml of 20 mM of NADP solution and 0.76 ml of glass distilled water were taken and mixed. Then 0.01 ml of the glucose-6-phosphate (substrate) was added to the cuvette and was mixed well. The reading was taken at 30 sec interval up to 5 min at 540 nm in spectrophotometer. Tissue protein was estimated by the method of Lowry et al.30
Statistical Analysis
The results were expressed as the Mean±Standard error of mean (SEM). Statistical analyses of the collected data were done by one-way Analysis of variance (ANOVA) followed by multiple comparison t-test. Difference was considered significant when P<0.05. P values are taken in respect of vehicle control in all cases of toxin-treated group.
RESULTS
Effect of MT81 and Bz- MT81 on age of vaginal opening and appearance of first estrous
Table 1 shows the effect of MT81 and Bz-MT81 on age of vaginal opening and appearance of first estrous in immature female rat. At the beginning of the experiment all the rats exhibited stage of diestrus (anestrus) at the study of vaginal smear as all of them were immature. Compared with the control group, MT81 (Groups III and IV) and Bz-MT81 (Groups V and VI) exposure delayed the age of vaginal opening and appearance of first estrous significantly (p<0.001) in a dose-dependent manner. The rats treated with MT81 and Bz-MT81 showed continuous diestrus stage throughout the period of treatment. On the other hand, saline and vehicle control group animals showed the age of vaginal opening and appearance of first estrous comparatively earlier.
| Table 1: Effect of MT81 and Bz- MT81 on age of vaginal opening and appearance of first estrous in prepubertal female rat. |
| Group |
Vaginal opening
(age in days) |
First estrous
(age in days) |
| Normal |
47.28±0.53 |
63.3±0.55 |
| Vehicle control |
38.24±2.2 |
64.4±1.24 |
| MT81(5mg/kg) |
47.48±1.91* |
71.12±1.73* |
| MT81(7mg/kg) |
60.7±0.53* |
79.62±1.56* |
| Bz-MT81(7mg/kg) |
49.8±1.32* |
73.2±0.94* |
| Bz-MT81(9mg/kg) |
64.5±1.48* |
81.8±0.37* |
| No. of animals per group =10. Results are Mean±SEM. Probability values are given in asterisks. *indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group. |
Body weight and reproductive organ weights
The body weights of the prepubertal female albino rats were decreased significantly (p<0.001) in higher dose of MT81-treated (Group IV) and Bz-MT81-treated (Groups VI) rats. The weights of ovaries (both sides), uterus decreased (p<0.001) whereas that of adrenal glands increased in a dose-dependent manner (Table 2).
| Table 2: Effect of MT81 and Bz- MT81 on body weight, weight of ovaries, uterus and adrenal gland prepubertal female rats. |
| Group |
Difference in body weight(g) |
Weight of ovaries (both sides) (mg/100g body wt.) |
Weight of uterus (both sides) (mg/100g body wt.) |
Weight of adrenal (both sides)
(mg/100g body wt.) |
| Normal |
40.17±0.32 |
30.30±1.33 |
78.46±0.25 |
15.16±0.97 |
| Vehicle control |
39.304±1.42 |
30.84±0.34 |
77.48±0.62 |
15.80±0.29 |
| MT81(5mg/kg) |
25.454±1.51* |
24.66±1.96* |
49.58±0.87* |
17.10±1.53 |
| MT81(7mg/kg) |
16.50±0.37* |
18.50±0.32* |
37.12±1.07* |
19.46±0.70* |
| Bz-MT81(7mg/kg) |
29.664±1.48* |
27.14±0.62* |
52.72±0.64* |
18.60±1.37*a |
| B Bz-MT81(9mg/kg) |
18.556±1.56* |
20.52±1.15* |
39.36±1.14* |
24.12±1.59* |
| No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. *aindicates 0.02>p>0.01; *indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group. |
Effects of MT81, Bz-MT81 on ovarian and adrenal cholesterol and ascorbic acid content
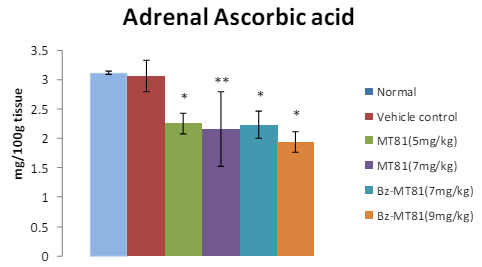
MT81 and its benzoylated analogue caused an accumulation of total cholesterol (p<0.001) and ascorbic acid in the ovary (Figures 1 and 2) of female prepubertal rats, whereas the cholesterol and ascorbic acid content of adrenal gland (Figures 3 and 4) were decreased.31
Figure 1: The bar diagram shows the effect of MT81 and benzoylated-MT81 on total cholesterol content in the ovaries of prepubertal female Wistar rat. No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. *indicates
p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
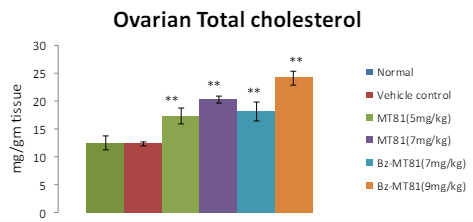
Figure 2: Shows the effect of MT81 and benzoylated-MT81 on total ascorbic acid content in the ovaries of prepubertal female Wistar rat. No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. **indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
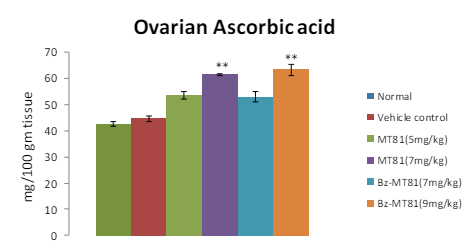
Figure 3: The bar diagram shows the effect of MT81 and benzoylated-MT81 on total cholesterol content in the adrenal glands of prepubertal female Wistar rat. No. of animals per group =10. Results are Mean±SEM. Probability values are given in asterisks. **indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
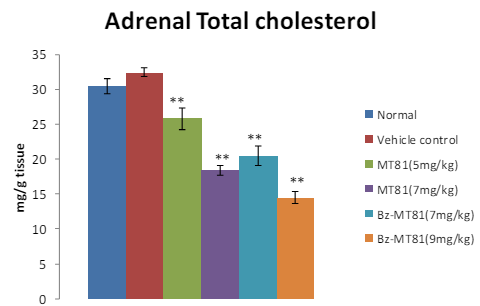
Figure 4: Shows the effect of MT81 and benzoylated-MT81 on total ascorbic acid content in the adrenal glands of prepubertal female Wistar rat. No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. *indicates p=0.001; **indicates p< 0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
Effects of MT81, Bz-MT81 on ovarian and adrenal ∆-3β HSD and G-6-P-D activity
The treatment of prepubertal female albino rats with MT81, Bz-MT81 reduced the activities of ∆-3β HSD and G-6-P-D (Figures 5, 6) enzymes in ovary and increased the activities of these enzymes (Figures 7, 8) in the adrenal gland.32
Figure 5: The bar diagram shows the effect of MT81 and benzoylated-MT81 on the activity of ∆5, 3β-hydroxy streroid dehydrogenase in the ovaries of prepubertal female Wistar rat. No. of animals per group =10. Results are Mean ± SEM. Probability values are given in asterisks. *b indicates 0.02> p>0.01; *c indicates 0.01> p>0.001; * indicates p=0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
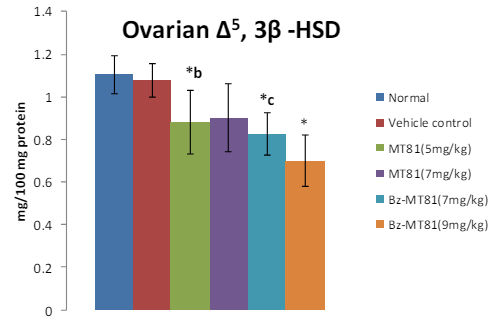
Figure 6: Shows the effect of MT81 and benzoylated-MT81 on the activity of glucose -6-phosphate dehydrogenase in the ovaries of prepubertal female Wistar rat. No. of animals per
group =10. Results are Mean ± SEM. Probability values are given in asterisks. *b indicates 0.02> p>0.01; *indicates p=0.001; **indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
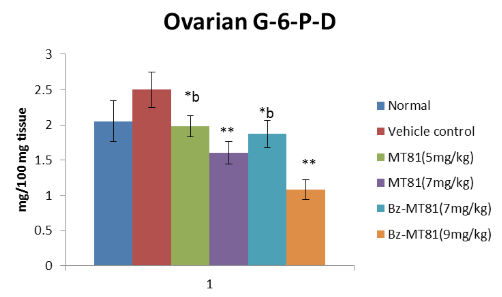
Figure 7: The bar diagram shows the effect of MT81 and benzoylated-MT81 on the activity of ∆5, 3β-hydroxy streroid dehydrogenase in the adrenal gland of prepubertal female Wistar rat. No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. *c indicates 0.01>p>0.001; *indicates p=0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.

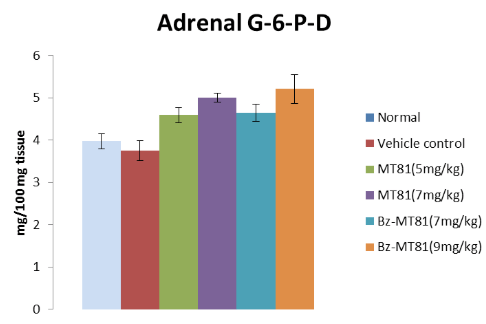
Figure 8: Shows the effect of MT81 and benzoylated-MT81 on the activity of glucose6-phosphate dehydrogenase in the adrenal gland of prepubertal female Wistar rat. No. of animals per group=10. Results are Mean±SEM. Probability values are given in asterisks. *indicates p=0.001; **indicates p<0.001. P values are taken in respect of vehicle control in all cases of toxin-treated group.
DISCUSSION
In case of prepubertal rats, administration of MT81 and Bz-MT81 remarkably delayed the onset of sexual maturity as evidenced by the age of vaginal opening and appearance of first estrus (Table 1).
The results of this investigation demonstrated the reduction in body weight and the wet weight of the ovary, uterus; that may be due to decreased anabolic role of estradiol on the body weight and wet weight of the ovary. This reduction may also be due to other toxic effects of MT81 and Bz-MT81 on other systems of the animal (Table 2). The disturbance in the reproductive cycle i.e. the delay in the age of vaginal opening and appearance of first estrus and the decrease in the weight of the ovary and uterus may be related with the reduction of ovarian steroidogenesis.
Cholesterol is as an obligatory precursor in progestin biosynthesis in rat, rabbit and bovine luteal tissues.33 Thus, in present study, the significant elevation in cholesterol content of ovarian tissue of MT81 and Bz-MT81-treated rats suggest the non-utilization of cholesterol towards biosynthesis of hormone in ovaries. Thus it results the hypofunctioning of steroidogenic activity of the ovary of the toxin treated rats. The accumulation of ascorbic acid in the ovaries of treated rats gives additional support to the inhibition of steroidogenic activity.
It is well documented that ∆5-3β HSD is an important key enzyme involved in steroid biosynthesis.34 According to Mckerns35,36 gonadotropins through the activation of glucose-6-phosphate metabolism in the pentose phosphate pathway increases the rate of production of NADPH essential for the hydroxylation reaction in the formation of steroid hormones from cholesterol. The reduction of cholesterol and ascorbic acid along with accompanying increase in the weight of the adrenal gland, the activities of the ∆5-3β HSD and G-6-P-D indicates the increased steroidogenesis in adrenal gland.
The importance of G-6-P-D from pentose phosphate pathway for the synthesis of estrogen in the sexually immature animals has been reported earlier.37,38
Anestrus vaginal smears and ovarian hypofunction appear to be due to the absence of or decrease in circulating gonadotropins. This is established by the regaining of the normal ovarian and uterine weights in starved animals following the injection of hypophyseal extract and chorionic gonadotrophin.39,40 Maximum synthesis and secretion of ovarian steroids take place in proestrus stages. The disturbances in the reproductive cycle and the decrease in the weight of the ovary and uterus in the present investigation may be related to the diminution of ovarian steroidogenesis.
The administration of MT81 and Bz-MT81 in prepubertal female rats resulted in decreased ∆5-3β HSD and G-6-P-D activities possibly due to the decrease in ovarian hormone production which was also seen in the effect of alpha and beta-zearalenol on the enzymatic activity of 3β HSD.41
Further support regarding the reduction of ovarian steroid hormone synthesis is evident from the accumulation of cholesterol and ascorbic acid in the ovary of toxin-treated rats.It is related to the hypofunctioning and non-functional ovary.7,42 The abovesaid effect was reversed in case of adrenal gland.
Therefore, in the present investigation a fall of ∆5-3β HSD and G-6-P-D of ovary after toxin treatment suggests that Bz-MT81 is less toxic than MT81 but its effect was more in case of the anti-fertility activity. From the above findings, it is evident that MT81 and Bz-MT81 cause prominent inhibition in ovarian steroidogenesis in a dose-dependent manner in prepubertal female Wistar rats and Bz-MT81 is more potent than its parent toxin MT81 as Bz-MT81 has less LD50 value compared to MT81.
ACKNOWLEDGEMENT
The authors are grateful to ICMR, New Delhi, India for providing financial support.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.














