INTRODUCTION
Cyanuric acid (CA) and its derivatives are heterocyclic compounds with a symmetric 1,3,5-triazine core. They are widely used in the chemical, pharmaceutical and textile industry.1,2 The applications of cyanuric acid (1,3,5-triazine-2,4,6-triol; 2,4,6-trihydroxy-1,3,5-triazine (Figure 1, A.)) span from disinfection of premises, textile and vessels to different fields of organic synthesis (production of antioxidants, cross-linking agents, glues, pesticides, herbicides, resins, bleaching agents and synthesis of antineoplastic drugs).1,3,4 Human exposure to cyanuric acid may be through swallowing swimming pool water, through drinking water processed from surface water, or through consuming fish, which may accumulate this chemical.5,6 It has been shown that CA has low acute toxicity in mammals. The oral LD50 in rats is greater than 10,000 mg/kg; the dermal LD50 in rabbits is greater than 7940 mg/kg.6 Also, in vitro studies on feline, canine and human kidney cell lines exposed to CA have demonstrated low-levels of toxicity.7,8 In addition, it is known that CA in combination with its structural analogue melamine, exert stronger cytotoxic effects in vitro than CA or melamine alone.9
Figure 1. Chemical Structures of s-triazine Derivatives
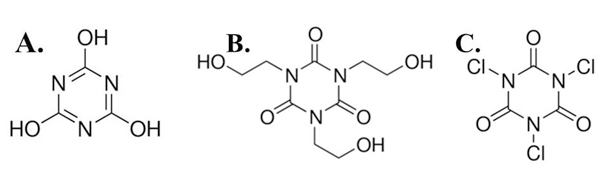
(A) cyanuric acid, (B) 1,3,5-tris(2-hydroxyethyl) isocyanurate, (C) trichloroisocyanuric acid.
1,3,5-tris(2-hydroxyethyl) isocyanurate (1,3,5-Tris(2-hydroxyethyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione; THIC) (Figure 1, B.) is a derivative of CA that is used for the synthesis of dyes, agrochemicals, pharmaceuticals and plasticizers, as well as in the production of heat resistant wire enamels, rubber materials, alkyd resins, urethanes, and polyesters. The toxicity of THIC has been studied in vitro on Chinese hamster ovary (CHO) cells using the chromosomal aberration test and the sister chromatid exchange assay. These tests revealed no cytotoxicity and negative effects.10 No data are available on the effects of THIC on humans or human cells in vitro.
Trichloroisocyanuric acid (1,3,5-trichloro-2,4,6-triazinetrione; TCIC) (Figure 1, C.) is a strong oxidizer and chlorinating agent. It is a bleaching and anti-shrinking agent, highly effective broad-spectrum disinfectant with excellent bactericide and fungicide properties.11 It is generally used for disinfection of water, recycling of industrial waters, disinfection of swimming pools, restaurants and other public places. In vitro studies on TCIC cyto- and genotoxicity towards the fish cell line RTG-2 have been performed showing that the compound induces DNA strand breaks and has the strongest cytotoxic effect compared to two other biocides.12 However, there are no reports about TCIC toxicity against human cells.
Considering the wide industrial application of CA, TCIC, and THIC, the data about their toxicity against different human cell types including cancer cells are limited. Therefore, to provide more information on the topic, the current study presents results from in vitro cytotoxicity screening using 4 human cell lines treated with different concentrations of CA, TCIC and THIC. Our data demonstrate that among the three triazines TCIC exerts the highest cytotoxicity. Interestingly, TCIC induces stronger inhibitory effects on cancer cells compared to normal fibroblasts, which suggest selective antitumor potential.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
The following human cell lines were used for evaluation of CA, THIC, and TCIC cytotoxicity: HeLa (ATCC CCL 2, NBIMCC 164) established from cervical adenocarcinoma; A549 (ATCC CCL 185, NBIMCC 2404) originating from lung carcinoma; CaOV (NBIMCC 1108) isolated from testicular cystadenoma, and a non-cancerous fibroblast cell line F derived from preputium.
All cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, 100 μg/mL streptomycin and 100 IU penicillin (all from Merck KGaA, Darmstadt, Germany) at 37°C, 5% CO2-atmospheric air mixture and high humidity. The cells were expanded in 75 cm2 culture flasks (TPP, Trasadingen, Switzerland). Prior to the in vitro cytotoxicity assays, the cells were detached from the culture vessel by trypsinization. The concentration of viable cells was determined and adjusted to 1×105 cells/mL for the MTT and Neutral red (NR) assays and 100 cells/mL for the clonogenic assays.
In vitro Cytotoxicity Assays
To characterize the cytotoxicity of CA, THIC, and TCIC in vitro, MTT, NR, and clonogenic assays were performed. For MTT and NR assays, 200 μL cell suspension/well were seeded on 96-well plates (TPP, Trasadingen, Switzerland) and cultured in standard supplemented DMEM for 24h at 37°C, 5% CO2/atmospheric air in a humidified incubator. Then, the culture medium was replaced with complete DMEM containing test-compound (i.e. cyanuric acid [C3H3N3O3], trichloroisocyanuric acid [C3Cl3N3O3] or 1,3,5-tris(2-hydroxyethyl) isocyanurate [C9H15N3O6] purchased from Merck KGaA, Darmstadt, Germany). CA, THIC, and TCIC were assayed in three different concentrations: 10 μg/mL, 100 μg/mL and 500 μg/mL. Ten mg/mL stock solutions of the triazines were prepared in Dulbecco’s phosphate buffered saline (DPBS) without calcium and magnesium chloride (Merck KGaA, Darmstadt, Germany). To achieve the assayed test-concentrations of all triazines the stock solutions were diluted in standard culture medium. The cells were cultured with CA, THIC or TCIC for 24 h and 72 h. Cyclosporin A (Novartis, Basel, Switzerland) served as a positive control for all in vitro cytotoxicity tests. The compound was assayed in the same concentrations as the triazines. All samples were plated in triplicates.
The principle of the MTT in vitro cytotoxicity assay has been described by Mosmann.13 For the present experiments, after incubation with triazines (24h, 72h), 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide provided by Merck KGaA, Darmstadt, Germany) solution was added to all samples. The reagent was diluted directly in the culture medium to a final concentration of 0.5 mg/mL. Then, the cells were incubated for 3 h in a humidified incubator at 37°C and 5% CO2-atmospheric air content. After the incubation period, the amount of formazan formed by MTT reduction and accumulated in viable cells was determined. For this aim, the MTT containing medium was removed and 100 μL DMSO were pipetted into each test-well. The cells were incubated for 15 minutes at room temperature on a shaker and then, absorbance at 570 nm wavelength was measured using Synergy-2 reader (BioTek, Winooski, VT, USA). Absorbance units from each test sample and from the control cells incubated in standard culture medium without triazines were used to calculate the percent inhibition of cell growth rate. Consequently, IC50 values were calculated.
Neutral red (NR) uptake assays were performed as described by Repetto.14 HeLa, A549, CaOV and F cells were expanded, detached, seeded on 96-well plates (TPP, Trasadingen, Switzerland) and treated with triazines in the same way as for the MTT assay. The NR assay enables evaluation of lysosome functionality and cell culture viability following treatment with test-agent.15 In our experiments, after 24 h and 72 h triazine exposure, the cells were stained with NR solution 0.5 mg/mL for 3 h at 37 ºC and high humidity. Then, the culture medium was aspirated and 100 μL solution containing 50% ethanol and 1% acetic acid was added to all samples in order to extract the stain accumulated in the cells. The culture plates were incubated for 15 min under continuous mild shaking and then absorption at 540 nm was measured using Synergy-2 reader (BioTek, Winooski, VT, USA). Percent inhibition of cell growth rate and viability was calculated based on the detected absorbance units. Accordingly, IC50 values were determined.
To perform clonogenic assays16 HeLa, A549 and CaOV single cell suspensions with concentrations of 100 cells/mL were seeded on 12-well plates using 0.5 mL/well (TPP, Trasadingen, Switzerland). After 24 h, triazine solution was added to the cells. The test-reagents were assayed in four different concentrations – 100 µg/mL, 50 µg/mL, 25 µg/mL and 5 µg/mL. The cells were cultured in supplemented DMEM containing triazines for 10 days until cellular clones were formed. Then, the culture medium was aspirated; the clones were washed with Dulbecco’s phosphate-buffered saline (DPBS) and incubated with Giemsa stain for 20 minutes. After that, the stain was washed away with distilled water and colored clones were counted. Clonogenic efficiency (CE) was calculated using the formula: CE= (Number of clones in the sample)/(Number of clones in the control )*100. This value was used to calculate percent inhibition of clonogenic efficiency: % inhibition=100-CE.
Measurement of Intracellular ATP Concentration
For assessment of intracellular ATP, HeLa, A549 and F cells in concentration 0,5×106 cells/well were seeded on 6-well plates (TPP, Trasadingen, Switzerland) and incubated under standard conditions for 24 h. Subsequently, the cells were exposed to 100 μg/mL TCIC for 24 h. After that, the cells were detached, washed with sterile DPBS and lysed. The intracellular ATP concentration was measured using a standard ATP Determination Kit (A22066, Molecular Probes, Life Technologies, USA) according to the manufacturer’s protocol. The assay was based on a bioluminescent reaction between D-luciferin and ATP catalyzed by the enzyme luciferase.17
Analysis of Cellular Morphology
The morphological analysis was performed on a standard inverted light microscope Inverso (Medline Scientific, Chalgrove, Oxon, UK) equipped with a high-definition digital camera Si-3000 and software (Medline Scientific, Chalgrove, Oxon, UK).
Statistics
Statistical significance of the results was determined by the non-parametric Mann-Whitney U-test using the StatView software (SAS Institute, USA). Calculated p values lower than 0.05 were considered statistically significant.
RESULTS
In vitro Cytotoxicity of CA, THIC, and TCIC Measured by MTT and NR Assays
Four human cell lines were used in the present study – three cancer cell lines derived from patients with different types of neoplastic lesions and a finite cell line established from normal dermal fibroblasts. This panel of distinct cell types allows evaluation of general cytotoxic effects, as well as potential antitumor activity. The results obtained by MTT and NR in vitro assays are present in Figure 2 and Figure 3, respectively. In these experiments, cyclosporine A, an antineoplastic and immunosuppressive agent, served as a positive control with strong cytotoxic effect. Cells cultured for the same time period in standard growth medium served as an untreated control. Percent inhibition of cell growth and development was calculated using the data from untreated cells and the ones exposed to a test agent. IC50 values for 72-hours exposure period were determined (Table 1).
| Table 1. IC50(µg/ml) According to Different in vitro Cytotoxicity Assays |
| Cell lines |
Test-compounds |
IC50 MTT assay* |
IC50 NR assay* |
IC50 Clonogenicity |
| A549 |
CA |
– |
– |
– |
| TCIC |
151.95 (±5.62) |
– |
7.95 (±1.24) |
| THIC |
– |
– |
– |
| CSP |
57.5 (±7.35) |
45.57 (±1.43) |
7.5 |
| HeLa |
CA |
383.64 (±3.21) |
447.37 (±3.25) |
– |
| TCIC |
80.01 (±2.65) |
– |
9.57 (±1.14) |
| THIC |
– |
– |
– |
| CSP |
56.7 (±3.57) |
51.31 (±5.87) |
7.5 |
| CaOV |
CA |
– |
– |
7.93 (±0.35) |
| TCIC |
326.13 (±7.35) |
– |
– |
| THIC |
– |
– |
– |
| CSP |
37.86 (±3.89) |
64.53 (±10.59) |
7.5 |
| Fibroblasts |
CA |
– |
– |
n.d. |
| TCIC |
– |
– |
n.d. |
| THIC |
– |
– |
n.d. |
| CSP |
54.7 (±6.35) |
89.2 (±7.32) |
n.d. |
*The values for MTT and NR assays are calculated using data from 72-hours exposition to triazines.
n.d. – not detected; CA – cyanuric acid; TCIC – trichloroisocyanuric acid; THIC – 1,3,5-tris (2-hydroxyethyl) isocyanurate; CSP – cyclosporine |
The MTT assay data demonstrate dose-dependent and time-dependent responses of the cells towards treatment with CA, THIC, and TCIC. Interestingly, the percent inhibition of cancer cell growth increased after longer exposition to triazines (Figure 2, B., D., F.), while normal fibroblasts demonstrated a very low -level of inhibition (on average 20%, 5% and 3 % for 24-hours exposition to CA, TCIC, and THIC, respectively) that did not increase with exposition time. Moreover, the NR assay data showed even the opposite tendency – inhibitory effects of CA and TCIC decreased fivefold and tenfold during the longer exposition test period (Figure 3, G., H.). In general, inhibition of fibroblast culture development was observed following CA and TCIC treatment. These effects were detected only by the NR assay (Figure 3, G.) suggesting lysosome-specific toxicity. However, the cells were able to surmount the negative influence because after longer exposition to the triazines (72 h) they showed lower percent inhibition (Figure 3, H.). On the other hand, stronger inhibitory effects of triazines against cancer cell lines were measured by the MTT assay. Only NR assays with HeLa cells showed pronounced percent inhibition (up to 45% for 72-hours exposition to TCIC, 65% after 72 h treatment with CA, 10% for 72 h exposition to THIC) comparable to the data from MTT assays (Figure 2, B., C., D., F.). In fact, among all cell lines, HeLa showed the highest sensitivity to triazine treatment. Comparing the inhibitory effects caused by the three test-compounds it is evident that TCIC is the most cytotoxic triazine with IC50(72) values in the range 80-330 μg/mL (80.01 μg/mL for HeLa, 151.95 μg/mL for A549 and 326.13 μg/mL for CaOV). Cytotoxicity ranking follows the direction TCIC>CA>THIC. THIC did not demonstrate marked inhibition on cellular growth and vitality. None of the triazines showed a stronger inhibitory effect than cyclosporine A.
Figure 2. Inhibitory Effects of Cyanuric Acid, Trichloroisocyanuric Acid and 1,3,5-Tris(2-Hydroxyethyl) Isocyanurate Measured by MTT Assay.
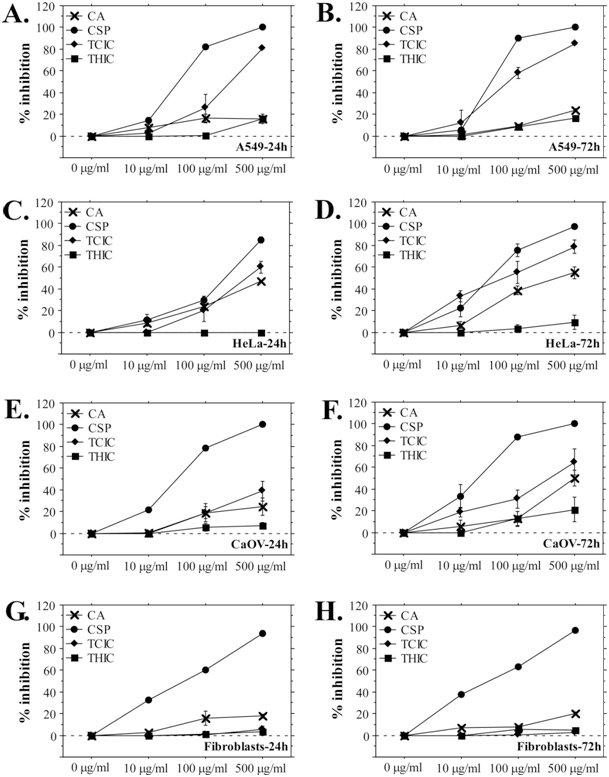
(A), (C), (E), (G) A549, HeLa, CaOV cells and fibroblasts were cultured in growth medium containing triazines for 24 h and analyzed by MTT assay; (B), (D), (F), (H) A549, HeLa, CaOV cells and fibroblasts exposed to triazines for 72 h. The results are shown as mean ± SE. CSP-cyclosporine A.
Figure 3. Neutral Red Assays with A549, HeLa, CaOV and Fibroblast Cells Exposed to s-triazines.
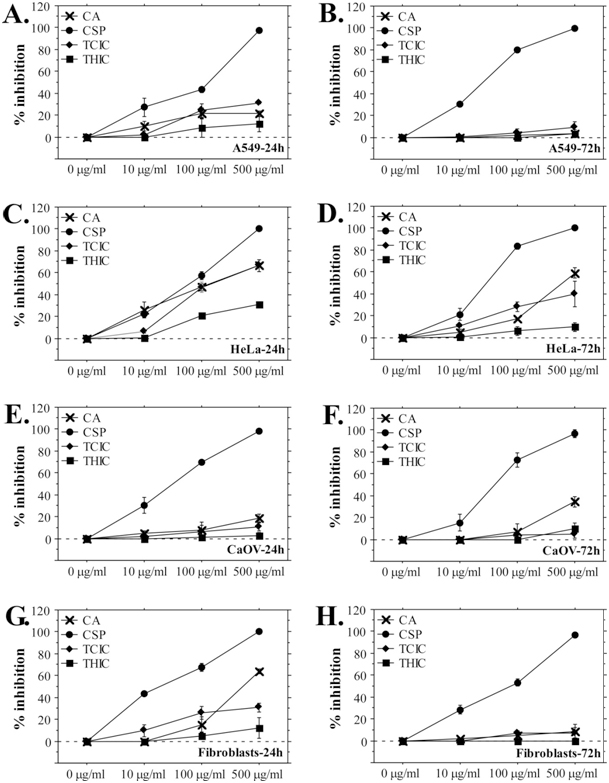
(A), (C), (E), (G) A549, HeLa, CaOV cells and fibroblasts treated with triazines for 24 h; (B), (D), (F), (H) A549,
HeLa, CaOV cells and fibroblasts after 72-hours exposure to triazines.
The results are present as mean ±SE. CSP – cyclosporine A.
TCIC Inhibits the Clonogenic Potential of Cancer Cell Lines
In addition to NR and MTT assays, the cytotoxicity of CA, THIC, and TCIC was analyzed by clonogenic assays. The aim of these experiments was to determine whether exposure to the triazines affects the proliferation potential of cells which corresponds to their clonogenic efficiency. Only cancer cell lines were used for these assays because normal fibroblasts exhibit very low clonogenic capacity. As shown on Figure 4, TCIC induced strong inhibition of the clonogenic efficiency of all cell lines that was similar to the effect of cyclosporine A. CA and THIC demonstrated significantly lower inhibition – 5 to 20% at the highest tested triazine concentrations. Calculated IC50 values based on the clonogenicity data highlight a considerable difference between TCIC toxicity (7-10 μg/mL IC50) and the other two tested triazines (CA and THIC with IC50 above 100 μg/mL) (Table 1). These data complement the results from MTT and NR assays and demonstrate strong cytotoxicity of TCIC on the level of proliferation capacity that is comparable to the effect of known cytostatic agents as cyclosporine.
Figure 4. Clonogenicity Inhibition of Cancer Cell Lines Induced by Cyanuric Acid and Its Derivatives.
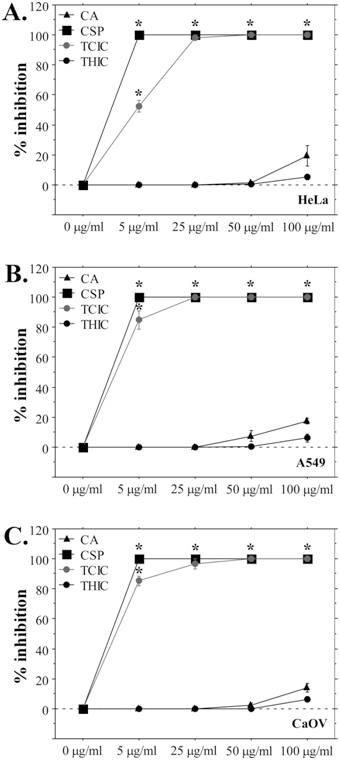
Clonogenic assay results for HeLa cell line; (B) A549 clonogenicity inhibition following exposure to triazines or cyclosporine; (C) Inhibition of clonogenic efficiency of CaOV cells after exposure to CA, THI, TCIC and CSP. The data are present as mean ±SE. *p<0.05.
Exposure to TCIC Affects Intracellular ATP Concentration
TCIC demonstrated the highest cytotoxicity among the three tested triazines. Thus, to investigate further its impact on cellular physiology, we measured ATP levels in HeLa, A549, and F cell lines. The cells were exposed to 100 μg/mL TCIC for 24 h. This concentration was chosen due to the striking difference in the data from MTT/NR assays and the clonogenic assays. While IC50 values calculated on the MTT and NR data were generally higher than 100 μg/mL, IC50 values based on clonogenicity assays were more than 10 times lower. The intracellular ATP levels of TCIC-treated cells were compared with results from control HeLa, A549 cells and fibroblasts grown in standard culture medium for the same test period (Figure 5). ATP concentration in all cell types exposed to TCIC was significantly decreased (p<0.05). TCIC-treated fibroblasts displayed slightly higher ATP levels than TCIC-treated cancer cells. This tendency supports the NR and MTT assay results that showed lower inhibition in normal fibroblasts.
Figure 5. Intracellular ATP Concentrations in Control Cells and Cells Treated with 100 µg/ml TCIC.
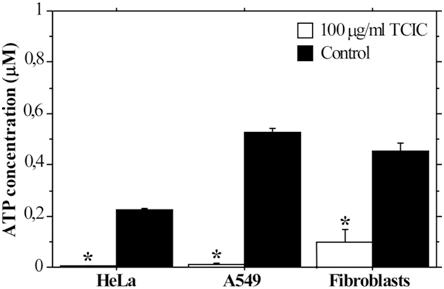
The cells were cultured in growth medium containing TCIC for 24h. The graph represent mean±SE. *p<0.05
TCIC Influence Cellular Morphology in vitro
Triazine-treated cells were analyzed microscopically in order to evaluate the impact of CA, TCIC, and THIC on cellular morphology. Pictures of A549 and F cells are present in Figure 6. Concordant with the results from MTT, NR and clonogenic assays microscopic analysis indicated a marked effect on the morphology of cells in culture after exposure to TCIC. The cell monolayers were disrupted; most of the cells shrank and showed abnormal morphology. This was evident when the cells were treated with 100 μg/mL TCIC. Lower triazine concentrations did not induce the notable effect. On the other hand, treatment with CA and THIC did not influence the integrity of cell culture monolayers and cellular morphology.
Figure 6. Effects on Cellular Morphology Observed Following Treatment with CA, THIC and TCIC.
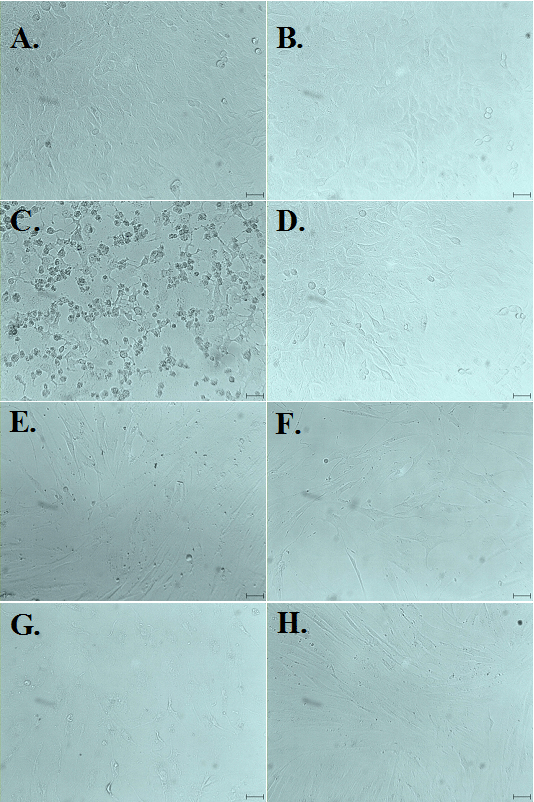
A549 cells and fibroblasts were treated with 100 µg/ml triazines for 24 hand then analyzed microscopically. The photos were taken during observation with 25x objective yielding 250 magnification. The black line on each photo indicates 10 m. (A), (B), (C) – A549 cells exposed to CA, THIC, TCIC respectively; (D) A549 control; (E), (F), (G) show fibroblasts treated with CA, THIC and TCIC accordingly; (H) control F culture.
DISCUSSION
CA, THIC, and TCIC belong to the group of 1,3,5-triazines (s-triazines) – one of the oldest known groups of organic compounds with unique chemical properties. The s-triazine structure is considered to be a remarkable tool in organic synthesis since it can take part in various types of interactions, particularly coordination, hydrogen bonds, electrostatic and charge-transfer attractions, and aromatic-stacking interactions.18 The symmetric structure of 1,3,5-triazines and their high affinity for binding to many enzymes makes them a suitable candidate for the production of compounds with therapeutic activity and various applications. Due to their unique pharmacologic properties, many s-triazine derivatives are widely used as chemotherapeutics, herbicides, pesticides, antibacterial agents, corrosion inhibitors, enzyme inhibitors etc. Cyanuric acid, trichloroisocyanuric acid, and 1,3,5-tris(2-hydroxyethyl) isocyanurate belong to this group of triazines with a vast application. The data about their toxicity to human cells is scarce. This fact motivated the present study, which provides new information concerning this field. Here, we demonstrate that TCIC has a high level of cytotoxicity with IC50(72)in the range 80-151.95 μg/mL, but CA and THIC exhibit low toxicity. THIC appeared to be the least toxic compound among the three tested triazines. Our data confirm previous reports on non-human cell lines showing low cytotoxicity CA and THIC (6, 8-10). Sanchez-Fortun et al. have investigated the cyto- and genotoxicity of TCIC in comparison with two other agents used for disinfection – Oxone® and sodium bromide. They report that TCIC induces DNA strand breaks at 1.2 mg/L when applied on the fish cell line RTG-2.12 Their results demonstrate a higher sensitivity of fish cells when they are compared with other biological material tested. For example, Ames test with Salmonella cells showed that TCCA does not induce gene mutations.19 Compared to the current study, RTG-2 cells again exhibit higher sensitivity to TCIC with 30.73 μg/mL IC50(48) determined by NR assay data.12 We were able to determine TCIC IC50 for human cell lines based on MTT assay results while the NR assays suggested higher than 500 μg/mL IC50(72).
The toxicity of cyanuric acid and mixtures of CA and melamine has been investigated using human embryonic kidney cells (HEK-293),9 Madin-Darby canine kidney cells and Crandell feline kidney cells,8 rat NRK-52E and human HEK293T cells.7 Consistent with our findings the reported data demonstrate low CA cytotoxicity with IC50 values higher than 1 mg/mL.7
In vitro evaluations of THIC toxicity to human cells have not been reported. A study with Salmonella typhimurium TA97, TA98, TA100, TA1535, and TA1537 indicates no cytotoxicity and negative effects of THIC in concentration 100-10000 μg/plate with and without activation.20 Sister chromatid exchange assays with Chinese hamster ovary cells at THIC concentrations of 0.402, 1.210, 4.020 μg/mL without activation and 0.381, 1.140, 2.290, 3.810 μg/mL with activation did not demonstrate genotoxic effect.10 Our data indicate very weak cytotoxicity of THIC against human cancer cell lines and no long-term toxicity to normal dermal fibroblasts, thus generally supporting the previous findings on other cell types.
The s-triazine ring provides convenient fundament for the development of biologically active compounds with lower toxicity and higher efficiency than other non-triazine heterocyclic compounds.21 Although to date, few compounds have reached the level of clinical trials, the perspective to find harmless agents with therapeutic potential, especially in cancer treatment, stimulates scientists’ efforts to search an appropriate compound in the s-triazine derivatives group. So far, research in this field has been focused on molecules with high molecular weight and complex structure. There is no information about potential anticancer properties of simple s-triazine derivatives that are important industrial tools with various applications including synthesis of antineoplastic agents. Therefore, our in vitro cytotoxicity evaluations included both cancer and normal cell lines enabling assessment of the potential anticancer effect of the studied triazines. Indeed, our data demonstrate the selective anticancer effect of TCIC. This triazine inhibited mitochondrial activity in cancer cells. Normal fibroblasts were not influenced significantly by TCIC treatment. The clonogenic efficiency of cancer cells was also profoundly affected.
Detailed structure-activity relationship (SAR) analyses of CA, THIC or TCIC have not been reported. However, it is clear that the cytotoxicity of these compounds is strongly dependent on their structure and chemical properties. The symmetric distribution of nitrogen atoms in the s-triazine ring facilitates the binding of different functional groups on 2, 4 and 6 position.2 The properties of each substituent and the special characters of the ring structure are responsible for the reactivity of different s-triazines. CA can bind up to three chlorine atoms and thus, forming TCIC. It has been suggested that TCIC could serve as a source of chlorine ions in situ which act as a catalyst for different reactions involving organic transformations. It is used mostly as a chlorination and oxidation agent.11 In vitro, these activities could affect and modify many cellular molecules hence, disrupting normal cell physiology and morphology. Therefore, the significant cytotoxic effects of TCIC could be attributed to its chemical structure and properties but additional experiments are needed to clarify the mechanisms of toxicity.
Different methods have been developed and applied for the evaluation of cytotoxic effects in vitro. For example, LDH assay, assays based on the reduction of tetrazolium salts, NR test, assays determining viable cell number and total protein or nucleic acid content, etc. Some of the most commonly used in vitro cytotoxicity assays were compared and it was suggested that MTT and NR tests exhibit the highest sensitivity.15 This accounted for the reason to use NR and MTT assays for the assessment of in vitro cytotoxic effects of CA, THIC, and TCIC. When these assays are applied on a panel of human cell lines, including normal and cancer cells, they allow discrimination of general cytotoxic effects, organelle-specific toxicity, and anticancer potential. However, to achieve a reliable estimation of these effects, it is necessary to perform several types of in vitro cytotoxicity assays measuring different cellular properties and functions. Therefore, in addition to MTT and NR assays, we conducted clonogenic tests in order to investigate the impact of triazine treatment on the proliferation potential of cells. Although clonogenicity evaluations have been initially used to estimate the effects of radiation on cells in culture, recently they are commonly applied to test cytotoxic and antitumor potential of different test-agents. These assays allow appraisal of the proliferative potential of cells reduced under the influence of the test agent.
The main reason for cell death is the loss of proliferative capacity. In reference to this, the cells that preserve their ability to divide indefinitely and generate a distinct clone are defined as clonogenic. The cells that keep their metabolic activity, divide once or twice, but cannot form a clone, are considered reproductively dead.16 The ability of a cell to develop and divide establishing a clone that could be visually detected proves its unaffected proliferative potential. In the clonogenic assays performed in the present study, the cells were exposed to a particular concentration of the test agent for a period of 10-days until clones were formed in the test-samples or the control. Therefore, these assays enable assessment of long-term inhibitory effects of triazines and proved the strong cytotoxicity of TCIC combined with significantly weaker negative effects of long-term exposition to CA and THIC. ATP measurements are concordant with these data – significantly reduced by TCIC exposure intracellular ATP invariably leads to proliferative inability. MTT and NR data also support these results showing that TCIC predominantly affects mitochondrial functionality which is related to ATP production as well.
Moreover, the present investigation proves the need to implement several different types of in vitro cytotoxicity assays in order to better characterize the inhibitory effects of test agent exposure. Hence, we were able to determine the mitochondria-specific toxicity of TCIC. Additionally, toxicity testing on a panel of cell lines allows identification of cell type-specific inhibition. Here, we show not only strong cytotoxic effects of TCIC but also demonstrate selective anticancer toxicity with HeLa cell line displaying the highest sensitivity.
CONCLUSION
The current study provides information about the cytotoxicity of s-triazine derivatives with a wide industrial application. We demonstrate that two of the tested agents – CA and THIC exhibit low toxicity to human cells, while TCIC markedly affected mitochondrial functions, cellular morphology and reproductive potential. The order of test-compound cytotoxicity in vitro is trichloroisocyanuric acid >cyanuric acid > 1,3,5-tris(2-hydroxyethyl) isocyanurate. Trichloroisocyanuric acid is more toxic to cancer cells than to normal fibroblasts, which indicates selective antitumor effect.
ACKNOWLEDGEMENTS
This work was supported by the Department for Scientific Research at the University of Plovdiv “Paisii Hilendarski” (Grant No. SP17-BF-010).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.












