INTRODUCTION
Citrus plants are popular worldwide due to their pleasant flavor and rich content of bioactive compounds, including phenolic compounds, terpenoids, vitamin E, vitamin C, and minerals.1 Sinetrol is a polyphenolic extract derived from citrus plants, which includes red-orange, sweet orange, bitter orange, grapefruit, and guarana. It is produced through physical methods such as cold pressing, extraction, and centrifugation.2 Sinetrol is rich in anthocyanins and flavonoids, with strong antioxidant, anti-inflammatory, blood glucose stabilization, and anti-obesity effects.3 Bergamot (Citrus bergamia R.) is also a type of citrus plant that grows in southern Italy. It mainly contains flavonoids such as naringin, neohesperidin, and neoeriocitrin.4Recent studies have shown that bergamot polyphenols exhibited antioxidant responses in vitro and in vivo and reduced glucose, cholesterol, serum triglycerides, systemic inflammation, and improved endothelial function.5In addition to their health properties, which include anti-inflammation, anti-obesity, and anti-oxidant effects, citrus plants have also been found to confer neuroprotective effects in dementia models, including Alzheimer’s disease (AD).6
Tea (Camellia sinensis (L.) O. Kuntze), originating from southern China with a history spanning over a millennium, is a popular non-alcoholic beverage. Presently, over 3 billion people worldwide use to consume tea.7 Based on its processing methods, tea can be categorized into white, yellow, green, oolong, black, and dark tea. Among them, green tea has the lowest volatile content (20 μg/g), in comparison, black tea has the highest (710 μg/g).8 Green tea, known for its potent antioxidant properties, is rich in catechins (flavon-3-ol) family, including (−)-epigallocatechin gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG), and (−)-epicatechin (EC). Besides, green tea contains abundant flavonoids, polyphenols, phenolic acids, amino acids, proteins, and lipids, making it crucial in promoting metabolism, anti-cancer effects, anti-inflammatory properties, anti-microbial activity, and cardiovascular protection.9
The prolonged intake of a diet with excessive caloric content leads to insulin over-secretion and the development of insulin resistant.10 Previous studies have indicated that chromium supplementation enhanced insulin sensitivity, promoting the metabolism of carbohydrates and fats, thereby benefiting the stabilization of blood sugar levels and the prevention of obesity.11 Chromium is a trace element in the human diet, and approximately 1% to 2% of chromium can be absorbed from foods such as meat, cereal, nuts, grains, molasses, and brewer’s yeast.12 The chromium yeast, known for its non-toxic safety, simple processing, and low production costs, is commonly used in dietary supplements.13
Obesity is the root cause of metabolic disorders such as type 2 diabetes mellitus (T2DM), non-alcoholic fatty liver disease (NAFLD), and cardiovascular diseases (CVD). Moreover, the high oxidative stress resulting from obesity lead to immune dysregulation, increasing the risk of cancer and autoimmune diseases.14 Obesity is not only prevalent in adults. Over the past 30 years, the incidence of obesity in children and adolescents has also doubled and quadrupled, respectively.15 While it is worth mentioning that weight loss in obese individuals can reduce the risk of mortality and various diseases. A weight reduction of 13% in obese individuals has been shown to decrease the risk of T2DM (41%), sleep apnea (40%), hypertension (22%), dyslipidemia (19%), and asthma (18%).16 This underscores the importance and necessity of weight loss for overall health.
Numerous studies had suggested that supplementation with plant extracts was beneficial for improving obesity. The dietary intake of a blend of bioactive compounds, aimed at targeting various mechanisms or enhancing their efficacy through synergistic interactions, has emerged as an innovative strategy for preventing and treating obesity.17,18 Thus, this study aims to develop an antiobesity dietary supplement combining Citrus extract (Sinetrol and bergamot) with green tea extract and yeast chromium. A 4-week supplementation trial was conducted on obese rats induced by a high-fat diet (HFD), and the changes in body weight, fat weight, and liver fat accumulation were analyzed.
MATERIAL AND METHODS
Experimental Supplement
The vegan complex (VC) supplement was provided by HealthTake Co., Ltd. (500 mg per tablet). The formula contained Sinetrol extract (polyphenols from citrus and guarana), green tea extract, Citrus bergamia extract, and chromium yeast. The VC supplement was prepared as a suspension in 0.5% carboxymethylcellulose (CMC) solution at three concentrations of 20.7, 41.4, and 82.8 mg/mL, administered at a volume of 1 mL per 100 g of body weight. The control group rats were provided with an equivalent volume of CMC solution.
Animal and Study Design
Male SD rats were obtained from BioLASCO Co., Ltd. (Taipei, Taiwan). A total of 70 rats, aged 6 weeks, were housed at the Animal Facility of China Medical University. The study was approved by the IACUC of China Medical University (No. 2021-400). The animal facility maintained a temperature of 22±2 °C and a 12-hour light-dark cycle (lights on at 8 AM and off at 8 PM). All rats were freely allowed to access the experimental diet and sterilized water throughout the experiment. After one week of acclimatization, the rats were divided into a normal diet (ND) group and a HFD group with 12 and 58 rats, respectively. The ND group was fed a standard diet with a caloric content of 2.85 Kcal/g (Altromin 1320, Altromin Spezialfutter GmbH & Co. KG, ImSeelenkamp, Germany). The HFD group was fed an HFD, which had a caloric content of 5.24 Kcal/g and a fat content of 34.9% (D12492, Research Diet Inc., New Brunswick, NJ, USA). After five weeks of diet induction, 10 rats in the HFD group that were either underweight or overweight were removed. The remaining rats were then divided into four groups, each consisting of 12 rats. These groups were oral gavage daily with CMC (control) and VC supplement at 1X, 2X, and 4X doses (207, 414, or 828 mg/kg, respectively) once per day. After 4 weeks of treatment, the experimental rats were sacrificed under 4% isoflurane with fasting overnight before and blood was withdrawn from the abdominal aorta for biochemical analysis. Liver and adipose tissues were collected, rinsed, and weighed.
Plasma Biochemistry Analysis
The blood samples were centrifuged at 4700 rpm for 15 minutes to obtain plasma for biochemical analysis. The analysis of aspartate amino transferase (AST), alanine amino transferase (ALT), triglyceride (TG), uric acid, and creatinine was performed using a commercial assay kit (Roche, Rotkreuz, Switzerland) and measured with a serum biochemical analyzer (COBAS MIRA, NY, USA). Blood glucose levels were determined using the blood glucometer CareSens II (i-SENS, Inc., Wonju-si, Korea), which utilizes the glucose oxidase enzyme method for glucose measurement. Non-esterified fatty acid (NEFA) was measured using a commercial assay kit (Non-Esterified Fatty Acid, RANDOX, County Antrim, UK). The concentrations of total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), and high-density lipoprotein-cholesterol (HDL-C) were determined using commercial assay kits (Fortress Diagnostics Limited, Antrim, UK). Blood sodium and potassium concentrations were measured using commercial enzyme-based assay kits (Fortress Diagnostics Limited, Antrim, UK).
Liver Lipid Concentration Determination
Lipids were extracted from the liver using the method described by Folch et al.19 For the lipid extraction, 0.1 grams of liver tissue were taken and homogenized with 2 mL of Folch solution (chloroform: methanol=2:1). The mixture was allowed to stand at room temperature for 60 minutes, followed by centrifugation at 5000 rpm for 5 minutes. The upper liquid layer was transferred to a clean 1.5 mL centrifuge tube, and 0.2 mL of 0.9% NaCl was added to the liquid and mixed well. At this point, the liquid appeared cloudy. After centrifuging at 2000 rpm for 5 minutes, it separated into two layers. The lower layer was retained and dried with nitrogen at 55 °C, and then dissolved by adding 100 µL of a solvent (tert-butyl alcohol: triton X-100: methanol=2:1:1) and heating at 65 °C for 15 minutes. The concentrations of TC and TG in liver were determined using commercial assay kits (Roche, Rotkreuz, Switzerland).
Statistical Analysis
The experimental data were analyzed using a one-way analysis of
variance (one-way ANOVA). Post hoc analysis was conducted using Duncan’s multiple range test. And the Shapiro–Wilk test was used to test the normality of the data. Significant difference between groups was determined when p<0.05.
RESULTS
Effect of VC Supplement on the Body Weight in HFD-induced Obese Rat
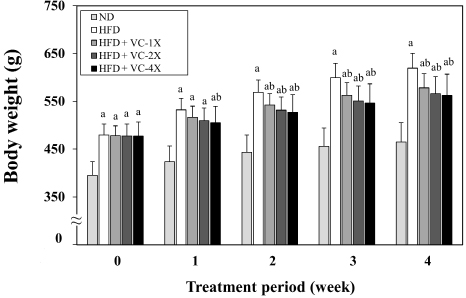
Figure 1 shows the body weight change during 4 weeks of VC supplementation. As results, the weight of rats fed with HFD was significantly higher than that fed with ND after 4 weeks of diet induction (week 0 of treatment period). After 1 week of treatment, the weight of the HFD+VC-4X group was significantly lower than the HFD control group. After 2 weeks of treatment, three VC treatment groups had significantly lower body weight than the HFD control group.
Figure 1. Effect of VC Supplement on the Body Weight in High-fat Diet-induced Obese Rats
Effect of VC Supplement on the Growth Parameters in HFD-induced Obese Rat
The growth parameters of rats during the treatment period are detailed in Table 1.
| Table 1. Effect of VC Supplement on Growth Parameters in High-fat Diet-induced Obese Rats |
|
Growth Parameters
|
ND |
HFD |
| Control |
VC-1X |
VC-2X |
VC-4X
|
| Induced BW (g) |
394.8±29.1 |
479.6±22.8a |
478.4±20.4a |
477.6±25.2a |
477.0±29.7a
|
| Final BW(g) |
464.8±40.5
|
619.3±31.2a |
578.2±29.4ab |
566.1±35.4ab |
562.0±44.6ab
|
| BW change (g) |
70.0±15.2
|
139.7±13.2a |
99.8±16.8ab |
88.5±17.4ab |
85.0±23.9ab
|
| Feed intake (g/rat/day) |
30.5±3.2
|
21.7±1.4a |
20.8±1.3a |
20.5±1.6a |
20.0±1.5a
|
| Energy intake (kcal/rat/day) |
86.8±9.0
|
113.6±7.4a |
109.6±6.9a |
108.0±8.6a |
106.4±8.1a
|
| Feed efficiency (%) |
8.1±1.2
|
23.0±1.3a |
17.1±2.5ab |
15.4±2.3ab |
15.0±3.5ab
|
| The reported values are the mean±SD (n=12). One-way ANOVA was performed for the data analysis. If there was a significant difference between the groups, Duncan’s test was used to compare means between specific two groups. A Significantly different from the ND group, p<0.05. b Significantly different from the group treated with HFD only, p<0.05. BW change=final B Winduced BW. Feed efficiency (%)=[Body weight change (g)/total feed intake (g)]×100%. BW, body weight. |
There was no significant difference in induced body weight between the four HFD-fed groups. However, after 4 weeks of treatment, the final body weight and body weight change were significantly lower in the three VC supplementation groups compared to the HFD control group. The feed intake and energy intake also showed no significant differences among the four HFD-fed groups, but feed efficiency was significantly lower in the three VC supplementation groups, demonstrating a dose-dependent effect.
Effect of VC Supplement on the Weight of Adipose Tissues in HFD-induced Obese Rat
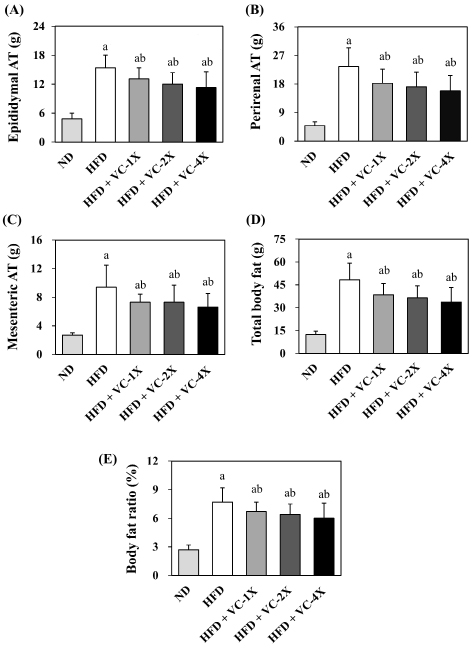
The results of the VC supplement on the weight of adipose tissues (A-D) and body weight ratio (E) are presented in Figure 2. After 4 weeks of treatment, the weight of epididymal adipose tissue, perirenal adipose tissue, mesenteric adipose tissue, total body fat, and body weight ratio all exhibited a significant decrease in the three VC supplementation groups when compared to the HFD control group.
Figure 2. Effect of VC Supplement on the Weight of Adipose Tissues (A-D) and Body Weight Ratio (E) in High-fat Diet-induced Obese Rats
Effect of VC Supplement on the Liver Lipid in HFD-induced Obese Rat
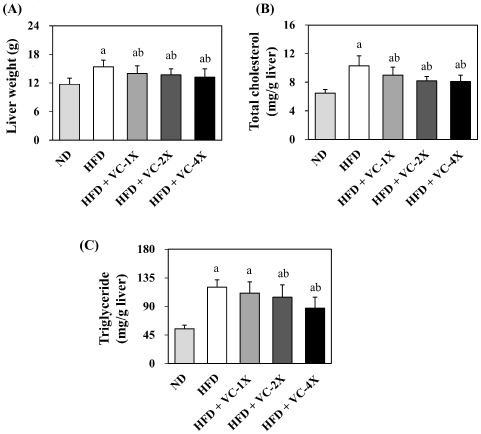
The livers of diet-induced rats were collected and weighed after sacrifice, and the lipid content of the liver was analyzed (Figure 3). The liver weight was significantly lower after 4 weeks of VC supplementation at three doses (1X, 2X, and 4X) compared to HFD-fed rats. In comparison to the HFD control group, the TC content in the liver significantly decreased in the three VC supplementation groups, and the TG content significantly decreased in the 2X and 4X VC supplementation groups after 4 weeks of treatment.
Figure 3. Effect of VC Supplement on (A) Liver Weight, (B) Total Cholesterol and (C) Triglyceride Level in High-fat Diet-induced Obese Rats
Effect of VC Supplement on Plasma Biochemical Parameters in HFD-induced Obese Rat
We analyzed the biochemical parameters related to liver function, kidney function, lipid profile, glucose, and electrolyte balance after 4 weeks of treatment. As shown in Table 2, the levels of AST, ALT, HDL-C, creatinine, uric acid, Na+ and K+ did not exhibit significant changes after three doses of VC supplementation (1X, 2X, and 4X) when compared with the HFD control group. However, the levels of TC, LDL-C, TG, and NEFA significantly decreased in the three VC treatment groups. Additionally, blood glucose content was significantly lower after 4 weeks of 4X VC supplementation.
| Table 2. Effect of VC Supplement on Serum Biochemical Parameters in High-fat Diet-induced Obese Rats |
|
Serum Biochemical Parameters
|
ND |
HFD |
| Control |
VC-1X |
VC-2X |
VC-4X
|
| AST (U/L) |
120.3±18.8
|
123.1±16.0 |
120.3±22.8 |
127.8±12.8 |
113.0±15.8
|
| ALT (U/L) |
39.7±6.9
|
45.3±16.2 |
37.0±9.5 |
36.5±10.0 |
37.5±14.0
|
| TC (mg/dL) |
65.2±11.2
|
79.0±10.2a |
64.8±16.2b |
62.9±12.3b |
66.4±16.2b
|
| LDL-C (mg/dL) |
18.5±4.2
|
26.7±4.3a |
19.3±7.8b |
16.8±4.8b |
18.8±6.9b
|
| HDL-C (mg/dL) |
36.5±6.3
|
39.8±4.3 |
34.7±6.3 |
34.7±6.3 |
34.4±8.2
|
| TG (mg/dL) |
34.3±3.7
|
46.0±4.2a |
36.9±7.0b |
36.9±6.0b |
35.0±8.3b
|
| NEFA (mg/dL) |
0.55±0.12
|
1.05±0.28a |
0.75±0.16b |
0.74±0.15b |
0.66±0.16b
|
| Glucose (mg/dL) |
127.9±21.7c
|
170.8±28.1a |
154.2±21.4a |
149.6±31.2 |
141.9±26.6b
|
| Creatinine (mg/dL) |
0.4±0.1
|
0.4±0.1 |
0.3±0.0 |
0.4±0.1 |
0.4±0.1
|
| Uric acid (mg/dL) |
0.7±0.2
|
0.8±0.3 |
0.7±0.2 |
0.8±0.3 |
0.7±0.3
|
| Na+ (mEq/L) |
142.3±1.9
|
140.7±2.6 |
140.8±3.4 |
141.2±3.1 |
141.5±2.0
|
| K+ (mEq/L) |
4.4±0.2
|
4.4±0.3 |
4.4±0.5 |
4.5±0.3 |
4.4±0.2
|
The reported values are the mean±SD (n=12). One-way ANOVA was performed for the data analysis. If there was a significant difference between the groups, Duncan’s test was used to compare means between specific two groups.
aSignificantly different from the ND group, p<0.05. bSignificantly different from the group treated with HFD only, p<0.05. AST: Aspartate amino transferase; ALT: Alanine amino transferase; TC: Total cholesterol; LDL-C: Low density lipoprotein-cholesterol; HDL-C: High density lipoprotein-cholesterol; TG: Triglyceride; NEFA: Non-esterified fatty acid. |
DISCUSSION
Obesity increases the risk of infectious and other chronic diseases, making it a highly prioritized issue. According to guidelines from the USA and the UK, a weight loss of 5% to 10% have a clinical health benefits.16 This study conducted an obesity animal model using a HFD-induction for 5 weeks, followed by a 4-week administration of VC supplement (207, 414, or 828 mg/kg) to investigate its antiobesity effects. The results revealed that rats induced with a HFD showed a 33.2% increase in body weight and a 289% increase in total body fat at the end of the trial compared to the ND group. Administration of 1X, 2X, and 4X the VC supplement significantly reduced the body weight (6.6%, 8.6%, 9.2%, respectively) and total body fat (20.2%, 24.6%, 30.2%, respectively) in obese rats (Figure 1 and 2). Previous study indicated that supplementation with Sinetrol (100 and 300 mg/kg) in leptin-deficient mice (ob/ob) for 7 weeks significantly reduced final body weight and weight gain. The 300 mg/kg of Sinetrol also significantly decreased the weight of abdominal adipose tissue, visceral adipose tissue, and epididymal adipose tissue.20 A Korean clinical trial also demonstrated that 12 weeks of Sinetrol polyphenol extract intake (900 mg/day) significantly reduced weight (3.28%), body fat (9.73%), waist circumference (5.71%), and hip circumference (4.71%) in obese subjects.21 Mice fed with an HFD containing 0.32% green tea extract exhibited significant reductions in both body weight and liver fat after 8 weeks, indicating a preventive effect against obesity.22 Rats administered with HFD combined 12% (v/v) juice of Citrus bergamia orally exhibited a significant decrease in the elevated body weight, visceral adipose tissue, as well as liver and heart weights induced by a high-fat diet.23 These studies suggest that VC supplement might exhibit an anti-obesity effect, as evidenced in various obesity animal models and obese patients.
Excessive calorie intake results in the storage of energy in the form of fat within the body. An HFD, due to its elevated calorie content, is prone to inducing weight gain. Moreover, when the body exhibits a higher efficiency in utilizing food, the probability of obesity tends to increase.24 In this study, the groups supplemented with VC supplement did not show alterations in feed intake and water consumption, yet demonstrated lower weight gain and higher feed efficiency (Table 1). Previous research has indicated that HFD-induced mice administered neohesperidin (50 mg/ kg) derived from bergamot exhibited significant reductions in body weight, adipose tissue weight, and serum inflammatory markers (tumor necrosis factor-alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1)) without feed intake change, demonstrating an effective inhibition of obesity.25 HFD-induced obese mice supplemented with green tea extract for 9 weeks (300 mg/kg) significantly downregulated the expression of genes associated with lipid synthesis in white fat tissue (SREBP1c, FAS, ACC1, SCD1) while upregulating genes related to thermogenesis (PGC-1α, UCP1) and fatty acid catabolism (HSL, ATGL, MGL).26 Another study revealed that supplementing guarana extract (1 g/kg) for 8 weeks in HFD-induced obese mice significantly reduced the body weight without altering feed intake. It also increased the content of mitochondrial functional proteins and the expression of genes related to thermogenesis (Sirt1, CREB1, AMPKα2, PGC1α, Nrf2, UCP1).27 These studies suggest that VC supplementation could mitigate obesity through the regulation of lipid metabolism and mitochondrial activity without affecting feed intake.
The liver is an organ responsible for fatty acid synthesis. Accumulation of excessive fats in the liver triggers inflammatory reactions and increase the risk of diseases such as hepatic lipid infiltration, non-alcoholic steatohepatitis (NASH), liver fibrosis, and liver cancer.28 Citrus plants contain abundant polyphenols that reduce the concentration of pro-inflammatory cytokines (TNF-α, interleukin 6 (IL-6), MCP-1, cyclooxygenase-2 (COX-2)) in the liver that inhibited HFD-induced non-alcoholic fatty liver disease (NAFLD).29 Previous studies have also shown that administering citrus extract (0.3% and 0.9% w/w of diet) to HFD-induced obese rats for 6 weeks decreased hepatic lipid, TG, and TC accumulation, and reduced hepatic acetylCoA carboxylase concentration.30 Similarly, Citrus bergamia, rich in polyphenols, reduced hepatic lipid accumulation, inflammatory cytokines (TNF-α, IL-6), and lipid oxidation concentration (malondialdehyde, MDA) in the livers of obese rats induced by a high sugarfat diet. Moreover, it increased the activity of antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT).31 A 12-week study administering green tea extract to HFD-induced mice also indicated that the abundant EGCG in green tea significantly reduced hepatic TC accumulation. This effect was achieved by suppressing the generation of lysophospholipids (LPLs), thereby reducing liver inflammation. As a result, the hepatic lipid metabolism of mice approximated that of mice fed an ND.32 In this study, rats administrated with VC supplement exhibited lower liver weight, hepatic TG, and hepatic TC levels (Figure 3). It suggests that VC supplementation not only possesses anti-obesity properties but also has a preventive effect against NAFLD, and the health benefits are associated with the abundant polyphenolic compounds present in the supplement.
HFD induction not only increases the weight of adipose tissue, leading to weight gain, but also causes abnormalities in lipid metabolism, increasing the levels of TC and TG in the blood.33 Previous studies have shown that supplementing citrus extract to rats for 42 days (0.25-0.75% of the diet) significantly reduced HFD-induced weight gain and hepatic lipid accumulation, exhibiting anti-obesity effects. It also regulated blood lipids by promoting the expression of genes related to cholesterol degradation, leading to a decrease in serum TC and LDL-C levels.34 In this study, rats supplemented with VC also exhibited lower-levels of TC, TG, LDL-C, and NEFA (Table 2). Furthermore, the 4X-VC supplement had a lowering effect on fasting plasma glucose (Table 2).
Previous study indicated that obese subjects, after supplementing with Sinetrol extract for 12 weeks (900 mg/day), experienced reductions in weight and body fat and showed a decrease in abnormal fasting blood glucose levels.21 Additionally, it increased the content of Apo A1 to promote lipid metabolism.35 In mice with HFD-induced obesity, supplementation with green tea extract for 6 weeks (0.1-1% of HFD) demonstrated anti-obesity effects, inhibiting the elevation of blood TC, TG, LDL-C, and glucose levels caused by HFD, and reducing liver inflammation.36 Mice subjected to a high-fat high-sugar diet with chromium yeast (32 mg/ kg) for a continuous 8 weeks exhibited a significant reduction in body weight and serum glucose levels, accompanied by an increase in hepatic glycogen accumulation, which improved insulin function and promoted energy metabolism.37 These studies collectively indicate that the effects of the VC supplement on the regulation of blood metabolic indicators are associated with its rich content of citrus, green tea extracts and chromium yeast.
This study confirmed the anti-obesity effect of the VC supplement, providing a new formula for weight control. The VC supplement can mitigate HFD-induced obesity by reducing the accumulation of fat tissue and liver lipids, however, the gut and neuron modulation of VC in the body and its effects in humans remain unclear. Therefore, further research is needed to investigate these limitations, and the findings of this study can serve as a dosage reference for future clinical trials.
CONCLUSION
In conclusion, supplementation with the VC for 4 weeks after inducing obesity with an HFD resulted in a reduction in the final body weight, weight change, feed efficiency, adipose tissue weight, and hepatic lipid accumulation in obese rats. This demonstrates the anti-obesity efficacy of the VC supplement, with a dose-dependent effect. Furthermore, the VC supplement administration also improved abnormal blood metabolic indicators. From the above, it could be inferred that the VC supplement is suitable for development as an anti-obesity dietary supplement, with an effective dose of 207 mg/kg rat, equivalent to a daily intake of 2000 mg of VC supplement for a 60 kg adult.
ACKNOWLEDGMENT
This research was founded by HealthTake Co., Ltd.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








