INTRODUCTION
Canine urolithiasis is a common cause of emergency urinary tract disease requiring a rapid definitive diagnosis for immediate surgical and/or medical therapy.1,2,3,4,5 Generally, dogs with urolithiasis are presented with serious clinical conditions such as occult haematuria and partial or complete obstruction. Haematuria, pollakiuria, stranguria, and dysuria are common clinical signs of lower urinary tract disease that are non-specific to cystic calculi.6 In dogs affected with urocystoliths, urocystoliths are difficult to palpate and physical examination findings are often normal unless the urethral obstruction is present, complete blood cell count (CBC) and serum biochemical analysis are usually normal and the clinical signs are not definitive.7 Reports indicate that haematuria can occurs in more than 50% of dogs with urinary bladder and renal neoplasia or other disorders are known to damage the mucosal surfaces of the urogenital tract such as infection, inflammation, trauma, vascular disease, and coagulopathies.8 Although haematuria is one of the most common clinical signs exhibited by dogs with urolithiasis, it can lead to wrong etiological diagnosis due to its multiple causes.
Thus, definitive diagnosis of urolithiasis cannot be made on history, clinical signs, haematology, urinalysis and other findings except with diagnostic imaging. Therefore, for dogs with lower urinary tract signs, imaging is crucial when clinical signs persist or recur and some of the breeds are susceptible to urolithiasis.6Survey and contrasts abdominal radiography, as well as ultrasonography, are often used to definitively diagnose and localize uroliths. Survey radiography and/or ultrasonography are the initial step in the sequential evaluation of the urinary system problems.9,10 Positive and/or negative contrast radiography is necessary to overcome inherent limitations of survey radiography to identify non-radiopaque uroliths and all free or attached soft tissue filling defects.9 Ultrasonographic evaluation of canine urinary system was found valuable in the diagnosis of, renal, bladder and urethral uroliths and bladder filling defects caused by neoplasia and granulomas except in the distal urethra.9,10 An ultrasound scan shows a clear hyperechoic area with acoustic shadows in all urinary bladder stones.8,9 Survey radiographs is an important tool to image the entire urinary tract and the complete length of the urethra to detect radiopaque urinary stones9,10 that should be used as a diagnostic complement to ultrasonography if the extent of urinary bladder disease cannot be adequately evaluated by ultrasonography.10
However, except a few retrospective studies reported on comparative canine urolithiasis detection capability of radiography and ultrasonography.11 Most of the studies conducted on canine urolithiasis were focused on the mineral composition and associated risk factors.2,5,6 Thus, this study was conducted considering the limited availability of recent information on the clinical haematological and microscopic urinalysis pictures at presentation and comparative diagnostic detection capability of ultrasonography and radiography in canine urolithiasis and/or concurrent urinary bladder growths.
MATERIALS AND METHODS
Study Animals
This study was conducted on 23 dogs affected with urolithiasis and presented to the Veterinary Teaching Hospital of Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab, India. About half of the animals were presented from Ludhiana and the remaining from various cities of Punjab State.
Clinical and Haematobiochemical Examination
Signalment
Age, breed, sex, neuter status and body weight of all the animals were recorded at the time of presentation.
History:
The features recorded for each case were the duration of the illness, the severity of the problem, history of the previous ailment, along with the medication, clinical symptoms such like anorexia, lethargy, depression, weight loss, vomiting, enlargement of the abdomen, polydipsia, polyuria, dysuria, haematuria, vaginal discharge.
Physical examination:
Respiratory rate (RR), pulse rate (PR), rectal temperature, colour of mucous membrane and hydration status were recorded. Abdominal palpation was also done.
Haematology:
Venous blood from cephalic/saphenous vein (2-3 ml) were collected in vials containing ethylenediamine-tetraacetic acid (EDTA), at the time of presentation and the relevant parameters were recorded including haemoglobin (Hb, g/dL) determined by acid haematin method using Sahli’s haemocytometer method and the value expressed in g%, total leukocyte count (TLC x103 per µL) evaluated using Neubar’s counting chamber method, packed cell volume (PCV, %) determined by Wintrobe method as described by Bellwood et al12 Differential leucocyte count (DLC, %) was determined as per method given by Harvey.13
Microscopic urinalysis:
Urine samples were collected aseptically in sterile syringes by cystocentesis and analyzed for microscopic examination. The urine sample was centrifuged for 5 minutes at 2000 rpm after mixed well in a graduated conical tube. The supernatant was discarded leaving behind the sediment. A drop of the sediment was then transferred onto a microscopic slide and covered with a coverslip and examined first under a light microscope at low power (10X) to assess the quantity and type of casts, cells, and sediments. Then samples were examined under high power (40X) to identify any abnormal structures.
Radiography:
All animals were subjected to radiography using the 160 mA X-Ray machine. Right lateral survey abdominal radiographs were taken for visualization of uroliths in the urinary bladder, urethra, and kidney while additional ventrodorsal views were taken for imaging kidneys. Radiographic factors given were 60-80 mAs and 70-95 kvp at a focal film distance of 32 inches. Potter Bucky grid and high speed intensifying screen were used. Plain or negative contrast cystography was done as per standard procedure is given by O’Brien.14 When plain radiography, failed to reveal a detectable abnormality in the urinary bladder in dogs presented with clinical signs of urinary tract affection, pneumocystography was employed using atmospheric air after aseptically passing catheter into the urinary bladder.
Ultrasonography:
In the present study, ultrasonography was carried out using a Concept/MVC veterinary ultrasound Scanners (Dynamic Imaging Limited, 9 Cochrane Square, Brucefield Industrial Park, Livingstone, Scotland, UK), with 3.5 MHz micro convex or 7.5 MHz linear array transducers. The images were recorded on thermographic printing paper of UPP-110 S series (Sony Corporation, Tokyo, Japan) with UP-595 CE (Sony Corporation, 6-7-35 Kitashinagawa, Shinagawa-Ku, Tokyo, Japan) video graphic printer for later reference.
Animal preparation and ultrasound scanning procedure:
The body area from the costal arch to the pelvic inlet was prepared by clipping and shaving hair and by clearing any grease or dirt for ultrasonographic examination. A coupling medium (Gel) was applied liberally over the area to increase the skin transducer contact. The animals were restrained in dorsal, right or left lateral recumbency as per the requirement on a padded table. The transducer of the appropriate frequency was selected. The machine gains were set appropriately and reset while scanning with a different probe. Scanning was carried out in a low light room with the scanner placed in such position that the screen could be viewed without altering its position in relation to the animals.
Ultrasonographic scanning was done following a systematic approach with the animal in the dorsal or lateral recumbency as desired. The examination was started at the cranial aspect of the abdomen by evaluating the liver and gall bladder and then preceded in a circular fashion around the left abdomen, next imaging the spleen, left kidney, urinary bladder and the prostate caudally. Similarly, the right kidney was scanned after locating the liver using liver and gall bladder as landmarks.
Organs of interest were scanned in transverse, sagittal and/or frontal planes to evaluate the internal architecture, boundaries/silhoutte, organ size, shape and position. The xiphoid cartilage, linea alba and pubis were used as the basic reference points. The landmarks were labeled and measurements, wherever required, were made with the help of in-built electronic calipers.
The amplitude of returning echoes (echogenicity) as visualized on two-dimensional, gray-scale images, were classified as increased (hyperechoic), normal (isoechoic), decreased (hypoechoic) or absent (anechoic) when compared with the normal echo amplitudes for that organ. Acoustic shadowing was used as a definitive or confirmatory diagnosis of uroliths.
Surgical procedure:
Cystotomy was performed in all dogs with urolithiasis in the urinary bladder and urethra except in four cases where retrograde hydropropulsion was failed and both cystotomy and urethrotomy was done in dorsal recumbency. Ventral abdominal area extending from xiphoid to the pelvic inlet was prepared for aseptic surgery. An intravenous cannula (Kit Kath, Hindustan Syringes and Medical devices Limited, Ballabgarh, India) was fixed in the cephalic vein and premedication was done with atropine sulphate (Atropine sulphate I.P., Jackson Laboratories Limited, Amristar, India) at 0.04 mg/Kg body weight and diazepam (Calmpose, Ranbaxy Laboratories Limited, Indore, India) at 0.5 mg/kg body weight administered slow IV. Five minutes later anaesthesia was induced with thiopentone sodium (Interval Sodium, RhonePoulenc (India) Limited, Bombay-25, India) (5% solution) “till effect.” The IV line was maintained with 0.9% normal saline solution (Sodium chloride injection LP., Punjab Formulations Limited, Jalandhar, India) at 10-12 mL/kg body weight per hour. Endotracheal intubation was done and animal was secured in dorsal recumbency. Maintenance of surgical plane of anaesthesia was done with thiopentone sodium (5%) IV or halothane. Surgical site was thoroughly scrubbed with chlorhexidine gluconate, cetrimide and isopropyl alcohol mixture (Aceptic. lCI India Limited, Chandigarh, India) (1:30 solution). Spirit was finally sprayed over the surgical site. Surgeons followed a routine scrubbing schedule.
For urethrotomy, after aseptic preparation, draping of surgical site was done and a urinary catheter was inserted through the penile urethra to the site of obstruction. Two to three centimeters ventral midline skin incision was made over the site of obstruction. Subcutaneous tissue was dissected to expose the retractor penis muscle, which was retracted laterally. With the penis stabilized in one hand, the corpus spongiosum urethra was incised on its exact midline over the site of obstruction to expose urethra. Then a longitudinal incision was made on the urethra. Once the urethra was entered the uroliths were removed and the catheter was gently extended through the urethral lumen into the bladder. Urethral incision was not closed. The skin incision was closed by simple interrupted or cross mattress suture pattern using nylon.
For cystotomy, after aseptic preparation, draping of surgical site was done. Following a ventral midline celiotomy, the bladder was exteriorized and abdomen was packed with sterile drape. The bladder was then drained through retrograde catheterization. About one inch cystotomy incision was made on the dorsal aspect of bladder wall in the least vascular area. The urocystoliths were retrieved with help of forceps/index finger. The lumen of bladder and the bladder neck was explored with index finger to detect any remaining uroliths. The catheter was then pulled back up to neck of bladder and retrograde flushing was done three to four times with sterile normal saline through the catheter to force any remaining urolith from bladder neck and urethra back into the bladder. The cystotomy incision was closed in two layers of continuous inverting suture pattern (Lambert followed by cushing) using No. 2/0 polyglactin 910 (Johnson and Johnson limited, Aurangabad, India). The abdomen was flushed with sterile normal saline. The abdominal wall was sutured in a single layer of interrupted suture pattern using No. 1 Vicryl. The subcutaneous tissue was sutured in a simple continuous suture pattern using No. 1 Vicryl. The skin incision was closed by interrupted horizontal mattress pattern using nylon.
Statistical Analysis
Quantitative data collected on signalment, vital signs, haematological parameters and microscopic urine analysis were summarized and simple arithmetic mean, standard error and 95% confidence interval were calculated. Mean values were compared by using student t-test. All the required statistical calculations were done using SPSS 16.0 statistical software.
RESULTS
Signalment: Age, Breed and Sex
Recorded signalment and vital signs of dogs affected with urolithiasis in the study are presented in Table 1. The mean and SE of the age of dogs suffering from urolithiasis was 5.4±0.5 years with 95% CI of 4.3-6.4 years. Amongst eight different breeds affected with urolithiasis in this study, the highest occurrence was recorded in German Shepherd, Spitz and Mongrel dogs (17% each) followed by Doberman and Labrador (13% each), Dalmatian and Pomeranian (9% each) and Boxer (5%). In this study gender wise occurrence of urolithiasis was found to be more in males with a ratio of 22:1.
| Table 1. Recorded Signalment and Vital Signs of Dogs Affected with Urolithiasis in the Study |
| Signalment and vital parameters |
Recorded Mean ± SE (95% CI) of the evaluated parameter |
| Mean age in years |
5.4±0.5(4.3-6.4) |
| Mean weight in kg |
23.7±1.9(19.6-27.7) |
| Male to female sex ratio |
22:1 |
|
Breeds
|
German Shepherd, Spitz and Mongrel (n=4, 17% each) |
| Doberman and Labrador (n=3, 13% each) |
| Dalmatian and Pomeranian (n=2, 9% each) and |
| Boxer (n=1, 5%) |
| Mean body temperature in °F |
102.2±0.2 (101.6-102.6) |
| Mean pulse rate in beats/min |
113±5(103-122) |
| Mean respiratory rate in breaths/min |
43±3(36-50) |
History and Clinical Signs
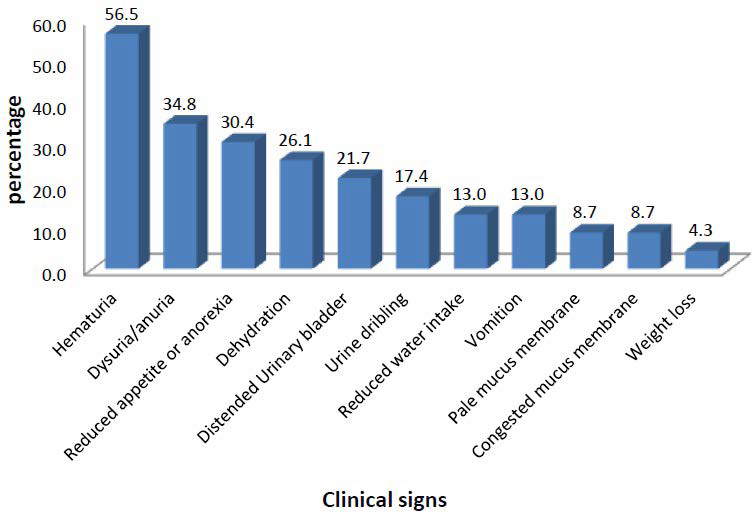
In this study, the most common clinical sign exhibited by dogs affected with urolithiasis were macroscopic haematuria (56.5%) followed by dysuria/anuria (34.8%), reduced appetite or anorexia (30.4%), dehydration (26.1%), distended urinary bladder (21.7%), urine dribbling (17.4%), reduced water intake (13.0%), vomiting (13.0%), pale mucus membrane (8.7%), congested mucus membrane (8.7%) and weight loss (4.3%) in decreasing order (Figure 1).
Figure 1. Clinical Signs Exhibited by Dogs Affected with Urolithiasis
Physical Examination
In urolithiasis affected dogs, mean and standard error (SE) of rectal temperature (°F), heart rate (beats/minute) and respiratory rate (breaths/minute) recorded were 102.2±0.2, 113±5 and 43±3 with 95% confidence interval of (101.6-102.6), (103-122) and (36-50), respectively, which were within the normal reference range. However, increased rectal temperature (>103°F) and heart rate (>120 beats/minute) were recorded in three and five dogs, respectively. In this study, distended urinary bladder was easily palpable in four dogs presented with complete obstruction.
Haematological Evaluation
Haematological evaluation of dogs affected with urolithiasis without obstruction versus partially or completely obstructed is presented in Table 2 below. The mean values of haemoglobin and packed cell volume (PCV) recorded in this study were within the normal range for both groups of dogs. The mean total leukocyte count (TLC) value was normal for unobstructed group while it was significantly elevated (p≤0.05) in partially plus completely obstructed group. The differential leukocyte count (DLC) (%) depicted relative neutrophilia in the case of unobstructed group while it showed absolute neutrophilic leukocytosis in partially plus completely obstructed dogs.
| Table 2. Recorded Haematological Findings in Urolithiasis Affected Dogs with and without Urethral Obstruction |
|
Type of Haematological examination
|
Urolithiasis without obstruction (n=7)
|
Urolithiasis with partial plus complete obstruction (n=15)
|
|
Mean ± SE (95% CI)
|
Mean ± SE (95% CI)
|
| Hb (g/dL) |
13.7±1.3a (10.3-17.0) |
14.5±0.4a (13.7-15.2)
|
|
PCV (%)
|
42.3±2.7a (35.6-49.0) |
41.1±1.1a (38.7-43.4)
|
|
TLC (x103 μL)
|
12.5±2.1a (7.4-17.6) |
21.1±2.8b (15.1-27.0)
|
| DLC (%): |
|
Neutrophil
|
77±3a (69-85) |
86±2b (82-90)
|
|
Lymphocyte
|
21±3(12-29) |
13±2(9-16)
|
|
Eosinophil
|
3±2(1-7) |
13±2(9-16)
|
|
Monocyte
|
0.3±0.3(0-1) |
0.4±0.3(0-1)
|
Microscopic Urinalysis
The result of microscopic urinalysis reveled haematuria (>10 RBC/HPF) in 18 (78.3%) of the dogs and pyuria (>5 WBC/HPF) in 11 (47.4%) (Table 3).
| Table 3. Abnormal Condition Observed During Microscopic Urine Examination in Dogs Affected with Urolithiasis (n=23) |
|
Abnormality
|
Number observed
|
|
Haematuria (>10 RBC/HPF)
|
18 (78.3%)
|
|
Pyuria (>5 WBC/HPF)
|
11 (47.8%)
|
|
Epithelial cells or casts (positive)
|
6 (26.1%)
|
|
Crystaluria (positive)
|
7 (30.4%)
|
|
Bacteria (positive)
|
4 (17.4%)
|
Radiographic and Ultrasonographic Evaluation
In this study, 19(82.6%) of the dogs had multiple uroliths and radiography diagnosed 19 of 21 urolithiasis cases in urinary bladder 2 of 2 in the kidney and 12 of 13 cases in the urethra while ultrasonography diagnosed 17 of 21 uroliths in the urinary bladder, one in the urethra, and none in the kidney. In this study, both ultrasonography and radiography failed to detect numerous small sized (1-5 mm) radiolucent uric acid stones in the urinary bladder of one dog but the largest of the stones that blocked the urethra in this same case was slightly radiopaque and detected by both radiography and ultrasonography. One radiolucent stone was detected by ultrasonography alone, but US failed to detect uroliths less than 5 mm in three other dogs. Among the 23 dogs diagnosed with urolithiasis, 13(56.5%) were in the urinary bladder and urethra, eight (34.8%) were in the urinary bladder alone, one (4.3%) was in the kidney alone and one (4.3%) was both in the kidney and urinary bladder. In the present study, from a total of 13 urethral stones seven (53.8%) were at the ischial arch, five (38.5%) were behind the os-penis and in one (7.7%) case uroliths were along the whole length of the urethra forming a chain. In the present study, the majority, (n=20, 87.0%) of the stones were radiopaque (++ to ++++) relative to the soft tissue density. In one case with numerous urate uroliths in UB were radiolucent except two found blocking the urethra while in remaining two (8.7%) cases they were slightly radiopaque (+).
From a total of 15 dogs that had either neoplastic growth and/or mild to severe cystitis concurrent with urolithiasis, ultrasound detected thickening of urinary bladder wall in six including dogs that had cystitis concurrent with urolithiasis (n=4), urolithiasis concurrent with transitional cell papilloma (n=1) and carcinoma in situ alone (n=1). In this study, the recorded mean and SE of urinary bladder wall thickness recorded by ultrasound in dogs with cystitis was 6.4±1.0 mm with 95% CI of (4.0-8.8 mm). The carcinoma in situ diagnosed by ultrasonography in this study was a misdiagnosis as ultrasound showed acoustic shadowing, which is a diagnostic characteristic of urolithiasis, but both plain and pneumocystography didn’t detect this case of carcinoma in situ.
In this study, both pneumocystography and ultrasonography diagnosed transitional cell papilloma that was concurrently presented with cystolithiasis while plain radiography was not diagnostic. In the case of transitional cell papilloma, diagnosed in this study, pneumocystography has revealed a mass grown from cranioventral part of the urinary bladder into the lumen whereas ultrasonography shown hyperechoic mass with thickening of urinary bladder wall (Table 4).
| Table 4. Number of Urolithiasis Cases and Concurrent Abnormalities Detected by Radiographic and Ultrasonography in the Affected Dogs |
|
Affection
|
Number of abnormalities detected |
| Anatomical location |
Radiography |
Ultrasonography
|
|
Urolithiasis
|
Renal (n=2)
|
2 |
0 |
UB (n=22)
[8 in UB alone, 13 in UB+urethra and 1 in UB+kidney] |
19 |
17
|
|
Urethral (n=13)
|
12 |
1 |
| Total (n=23) |
21(91.3%) |
17(73.9%)
|
|
Mild to chronic cystitis and urinary bladder (UB) growth concurrent with urolithiasis
|
UB (n=15) |
1(6.7%) |
6(40.0%)
|
DISCUSSION
Dogs affected with urolithiasis are often presented with nonspecific clinical signs of urinary tract affection that requires running multiple diagnostic tests. Uroliths can cause partial or complete urinary obstruction of the urethra leading to emergency situation that requires rapid detection and removal to avoid life threatening conditions such as postrenal azotemia, urinary bladder rupture and uroabdomen. In this respect, radiography and ultrasonography are excellent diagnostic tools for detection of urinary calculi each with its own complementary superior aspects and limitations. In addition to the diagnostic imaging recommended for definitive diagnosis and confirmation of uroliths, routine clinical, physical, haematological and urine examinations are also highly valuable to detect underlying concurrent disease processes occurring with urolithiasis and/or consequent to it.
Signalment: Age, Breed and Sex
The mean age of dogs suffering from urolithiasis in this study was 5.4 years. This is in agreement with previous reports that indicated dogs suffering from cystolithiasis and/or urethral calculi were above 4.8-year-old depending on the chemical composition of uroliths.2,4,15,16 Eight different dog breeds were affected with urolithiasis; the highest occurrence recorded in German shepherd, Spitz and Mongrel dogs in this study. The association of cystic and urethral urolithiasis with a variety of breeds was reported by several previous studies.2–5,15-17 Variation in the pathogenesis of urinary stones between species, breeds, genetics, metabolism and nutrition was also documented3 where nutrition is implicated as the major factor responsible for formation of uroliths in dogs and cats.6 Toy and small breed dogs were significantly associated with calcium oxalate urolithasis while struvite uroliths tended to be over-represented in medium and large breed dogs.4 The variation in the frequency of occurrence of urolithiasis in different breeds in the present study as well as previous reports may also be attributed to the changing trend in the preference of dog owners to different breeds of dogs. In this study gender wise occurrence of urolithiasis was more in males with a ratio of 22:1. Similar predominant occurrence of urolithiasis in male than female dogs was reported by previous studies.1,2,16,19 This is also in agreement with higher occurrence of urolithiasis reported in male equines and bovines.20,21 This high occurrence in male than female animals is likely due to the anatomy of the urethra, which is short and wide in females that may allow voiding most uroliths before obstruction and subsequent recognition of clinical signs as opposed to the male urethra, which is long with curved path and distally surrounded by ospenis predisposing dogs to frequent obstruction.1,2,21 However, previous reports showed female dogs predominating males in the occurrence of struvite urolith.2,4,5 The reported higher occurrences of struvite containing urolithiasis in female dogs was ascribed to infection-induced nature of struvite stones to which female dogs are at greatest risk due to their short and wide urethra.2
History and Clinical Signs
The most common clinical sign exhibited by dogs affected with urolithiasis in this study were haematuria, dysuria, reduced appetite or anorexia, dehydration, distended urinary bladder, urine dribbling, reduced water intake, vomiting, pale mucus membrane, congested mucus membrane and weight loss in decreasing order of occurrence. The clinical signs exhibited by the dogs in this study were in accordance with earlier studies of canine urolithiasis.6,22-24 The observation of haematuria and dysuria as the most frequent clinical signs in canines affected with urolithiasis are most probably associated with irritation of bladder mucosa and blockage of the urethra caused by uroliths, respectively.
In this study, the mean recorded values of vital signs were within the normal reference range except an increased rectal temperature (>103 °F) recorded in three dogs and increased heart rate (>120 beats/minute) in five dogs. The average respiratory rate recorded in affected dogs in this study was above the normal reference resting respiratory rate documented by Aiello et al.25 This is in agreement with the reported near normal rectal temperature and pulse rate but markedly elevated respiratory rate in dogs presented with urolithiasis.17
Haematological Evaluation
In this study, the recorded mean values of haemoglobin and packed cell volume (PCV) were within the normal range for all dogs, but the mean TLC was significantly elevated (p≤0.05) in partially and completely obstructed dogs. The DLC (%) depicted relative neutrophilia in the case of unobstructed group while it showed absolute neutrophilic leukocytosis in partially and completely obstructed dogs. This is in concordance with presurgical normal values of haemoglobin and PCV reported by previous studies in dogs with urolithiasis.16,19 The observed neutrophilic leukocytosis in obstructed dogs evaluated in this study may be due to uremia, stress and inflammation associated with the obstruction or mucosal damage caused by uroliths. Both acute and chronic inflammations are recognized common-causes of leukocytosis with neutrophilia in dogs.26 Pre-surgical elevation of TLC recorded in this study is in agreement with previous reports.16,18,23,24 In the DCL, the relative neutrophil and lymphocyte count were significantly different (p≤0.05) in dogs with partial and complete obstruction compared to unobstructed dogs. This is in agreement with presurgical neutrophilia and lymphopenia reported by previous studies in canine urolithiasis at presentation.16,23,24 Lymphopenia in cases of urethral obstruction might be due to stress and/or infection associated with the urolithiasis. Concurrent lymphopenia and eosinopenia are typical owing to release of endogenous corticosteroids in response to stress superimposed on the inflammatory neutrophilic leukogram.26
Microscopic Urinalysis
In this study, clinically occult haematuria was seen in 56.5% of dogs affected with urolithiasis while microscopic urine examination revealed subclinical microscopic hematuria (>10 RBC/HPF) in 18(78.3%) of the same dogs. This shows the importance of microscopic urine examination in order to detect subclinical microscopic hematuria that could be missed in gross urine examination to undermine the problem. A recent retrospective study also reported microscopic haematuria in 80.7% of urolithiasis affected dogs.11 Pyuria (>5 WBC/HPF) is recorded in 47.4% of dogs in this study. The pyuria may be caused by urinary tract infection (UTIs) s that might have caused some of the uroliths or the uroliths may have also predisposed the patient to a bleeding and/or UTI leading to pyuria. Association of infection with the most commonly analyzed struvite uroliths (43.8%,7,287/16,647) was documented.2 The severe injurious effect of uroliths is evident from the blood bathed rough cystoliths recovered from urinary bladder of affected dogs in this study that may lead to pyuria as a result of blood leak into urine in the bladder. Although, all the dogs evaluated in this study, had urolithiasis, urine crystals were detected only in seven (30.4%) dogs. This is agreement with the low detection of crystalluria (28.0 to 38.1%) reported by previous studies in urolithiasis affected dogs.11,16,23 This indicates that crystalluria is not a consistent finding in microscopic urinalysis of urolithiasis affected animals that could not be relied on as a diagnostic method for urolithiasis.
Radiographic and Ultrasonographic Evaluation
In this study radiography diagnosed 19 of 21 urolithiasis cases in urinary bladder, 2 of 2 in the kidney and 12 of 13 in the urethra while ultrasonography diagnosed 17 of 21 uroliths in the urinary bladder, one in the urethra, and none in the kidney. Thus radiography showed better capability in urolithiasis detection in the kidney, urinary bladder and urethra while ultrasonography was weaker in detecting uroliths in the kidney and urethra, but equally capable in detection of urolith in the urinary bladder. The ultrasonographic appearance of radiopaque or radiolucent renal calculi is described as showing bright echoes and acoustic shadowing.27 But ultrasonography didn’t show the characteristic acoustic shadowing in both renolith cases in this study, except revealing renal calcification characterized by increased focal parenchymal echogenicity in one case and hyperechoic medulla with loss of corticomedullary junction, both of which were in conclusive and non-diagnostic of renolith. In this study, ultrasonography was also better than plain and negative contrast cystography in the detection of cystitis and urinary bladder growths that were present concurrent with urolithiasis or alone. However, we cannot aver that ultrasonography less capable than radiography in the detection of either renal or urethral urolith since the number of renal urolith cases were very few in this study and ultrasonography has known inherent limitation for imaging the urethra due to the presence of pelvic bones in that anatomic area limiting the passage of ultrasound beam. It was opined that urethral calculi are difficult to visualize with ultrasound unless they are lodged near the neck of the bladder.6
In this study, both ultrasonography and radiography failed to detect numerous small sized (1-5 mm) radiolucent uric acid stones in the urinary bladder of one dog. According to Gatoria et al28 majority of urate uroliths were not radiolucent due to minor proportion of calcium phosphate content and29 also concluded that radiodense urocystoliths less than 3 mm in size and radiolucent uroliths were difficult to detect by survey radiography. In this study, however, in one male dog both plain radiography and ultrasonography diagnosed two moderately radiopaque ammonium acid urate urethrolith, in a case from which numerous small ammonium acid urate urocystoliths were retrieved during cystotomy. This shows that urate uroliths can be detected by radiography when the sized is large enough. It is opined that except for single, very small stones, cystic calculi are easy to detect sonographically regardless of their radiopacity.30
In this study, 82.6% of the dogs had multiple uroliths. Similarly occurrence of multiple uroliths was reported in 66.7% of studied dogs.23 In this study, 60.9% of dogs had stones in multiple anatomical locations with more common occurrence in the urinary bladder and urethra. This finding is in agreement with previous studies that reported more common occurrence of uroliths in the urinary bladder followed by the urethra in dogs, cattle and horses by Osborne et al.1,3,4,20,21,23,28,31 Reports showed the ventral groove of the ospenis is the most common site of urethral obstruction (66.7%) followed by post-ospenis (25%) and ischial arch region (8.33%).16,32 The long curved path of the urethral and the presence of ospen is surrounding the distal part makes urethra less distensible and more prone to obstruction creating acute emergency condition in dogs.
In the present study, the majority, (n=20, 87.0%) of the stones were radiopaque (++ to ++++) relative to the soft tissue density. In one case with numerous urate uroliths in UB were radiolucent except two found blocking the urethra while in remaining two (8.7%) cases they were slightly radiopaque (+). One report indicated that majority of uroliths were strongly radiodense (52.38%) followed by moderately radiodense (28.57%) and weakly radiodense (19.05%).28 This variation in this reported proportion of graded radiodensity of uroliths could be due to the subjective nature of grading the degree of stone density or variations of the radiation exposure factors used. The two most prevalent mineral types in cats and dogs are calcium oxalate and struvite uroliths comprised 80.8% in 75,674 uroliths analyzed in a recent study Houston et al4 and that both types of stone are generally radiopaque 6 explaining the reason for strong radiodense characteristics of most of the uroliths.
From 15 dogs presented with either neoplastic growth and/or cystitis concurrent with urolithiasis in this study, ultrasound diagnosed six cases, pneumocystogrpahy detected in only one case with transitional cell papilloma and plain radiography detected none. In the transitional cell papilloma, peumocystography revealed a mass grown from cranioventral part of the urinary bladder into the lumen whereas ultrasonography shown hyperechoic mass with thickening of the urinary bladder wall. In this study ultrasound also detected thickening of urinary bladder in five dogs and mean and SE of 6.4±1.0 mm (95% CI of 4.0-8.8 mm) bladder wall thickness was recorded. The thickness of the normal bladder increases with body weight and depends on the degree of distension where in the near-empty normal bladder, the mean wall thickness was 2.3±0.43 mm while in moderately distended urinary bladder (4 ml/kg) it was 1.4±0.28 mm.30 The mucosa of urinary bladder responds to inflammation or urinary tract infection by hyperplasia and hypertrophy, which causes thickening of the wall,33 that is easily detected in acute cystitis for the echogenicity of the bladder wall is hypoechoic due to edema whereas focal or diffuse thickening of the cranioventral bladder wall in response to chronic inflammation may be difficult to detect unless changes are dramatic.34
It was opined that diseases identifiable with survey radiographic findings are uncommon except cystic calculi and the inability of survey radiography compared to the detection capability of contrast cystography to diagnose intramural and intraluminal bladder tumors and other changes such as bladder wall outline, thickness, mural masses, and intraluminal defects was previously documented.35,36 The ultrasonographic appearance of the transitional cell papilloma seen in this study is analogous with stated observable focal hypoechoic mass in ultrasonographic imaging of urinary bladder is most likely a tumor.10 In this study, one carcinoma in situ showed acoustic shadowing, which is a diagnostic characteristic of urolithiasis.10 But no urolith was recovered during cystotomy except greatly swollen urinary bladder wall was observed associated with neoplastic growth. This case of carcinoma in situ is discussed here for its relevance as a false positive diagnosis of urolithiasis by ultrasonography otherwise it is not counted with the 23 urolithiasis cases. The case was later confirmed carcinoma in situ by histopathology using a tissue sample taken from the resected tumor during surgical treatment of the case. This showed that urinary bladder carcinoma may show acoustic shadowing in ultrasonographic evaluation leading to misdiagnosis of urolithiasis. Although, the possibility of false positive diagnosis was mentioned in the case of bladder tumors and polyps, they are not supposed to show acoustic shadowing, except hyper-echogenicity.10
In the present study, both radiography and ultrasonography failed to detect fibropapilloma, which was noticed only during surgery. This may be due to the small size of the fibropapilloma that was noticed during surgery. The size of urinary bladder tumor lesion was reported as an important determining factor in the rate of detection by ultrasonography such that the rate of detection of tumors less than 0.5 cm in diameter is less than 33.0% compared to 83.3% detection rate for tumors larger than 1.0 cm and 95.0% for tumors larger than 2.0 cm.10 It is opined that accuracy of sonographic diagnosis of urinary bladder tumor depends on the size of the mass where small masses of less than 0.3 cm, and those located in the trigone area are difficult to detect.34
CONCLUSION
The study showed that clinical signs as well as haematological and microscopic urinalysis findings can insinuate to urolithiasis, but they won’t conclusively enable to identify the etiology and localize the problem. The most common clinical signs exhibited by dogs affected with urolithiasis are haematuria and dysuria that should be taken as the leading clinical symptoms to consider urolithiasis in order to select an appropriate tool for further definitive diagnosis. Both ultrasonography and radiography are valuable and complementary diagnostic tools to confirm urolithiasis and concurrent cystitis and neoplastic growth in the urinary bladder. Radiography is relatively better in detecting uroliths in the kidney, urinary bladder and urethra with additional benefit of enumerating the number of uroliths enabling removal of all uroliths for best surgical management of the patient. Ultrasonography is superior in detecting urinary bladder inflammation and neoplastic growths with equal uroliths detection capability in the urinary bladder. However, ultrasonography is incapable to enumerate the number of uroliths and it is unsuitable for imaging the urethra due to pelvic bones. Therefore, both radiographic and ultrasonographic imaging is recommended for dogs presented with nonspecific signs of haematuria and dysuria for early confirmation and effective intervention.
DISCLOSURE
The approval of ethical committee was taken for conducting this study and followed all the animal ethics and welfare guidelines.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







