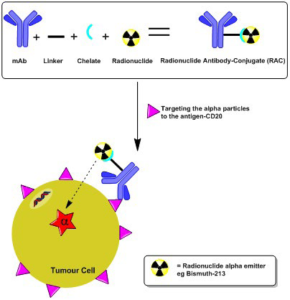
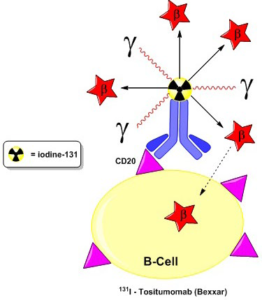
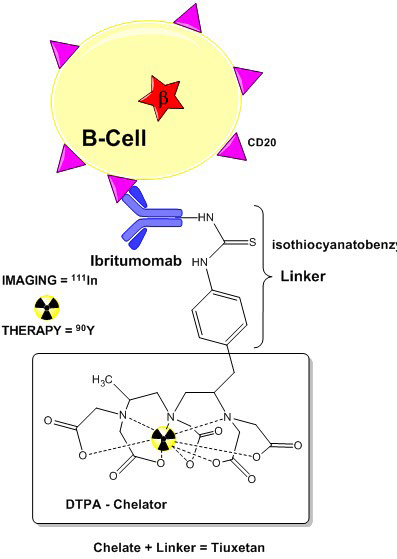
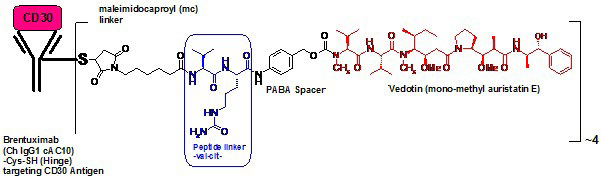
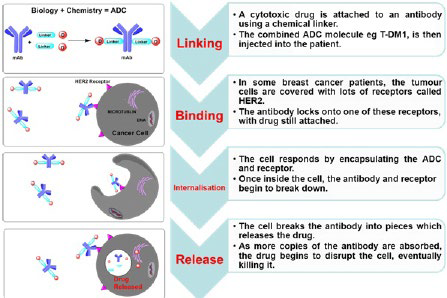
1. Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008; 8(6): 473-480. doi: 10.1038/nrc2394
2. Kitson SL, Quinn DJ, Moody TS, Speed D, Watters W, Rozzell D. Antibody-drug conjugates (ADCs) – A new generation of biotherapeutic bullets. Chim Oggi. 2013; 31(4): 30-36.
3. Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975; 256(5517): 495-497. doi: 10.1038/256495a0
4. Steiner M, Neri D. Antibody-radioniclide conjugates for cancer therapy: historical consideration and new trends. Clin Cancer Res. 2011; 17(20): 6406-6416. doi: 10.1158/1078-0432.CCR11-0483
5. Flygare JA, Pillow TH, Aristoff P. Antibody-drug conjugates for the treatment of cancer. Chem Biol Drug Des. 2013; 81(1): 113-121. doi: 10.1111/cbdd.12085
6. Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013; 64: 15-29. doi: 10.1146/ annurev-med-050311-201823
7. Rahman KM, James CH, Thurston DE. Effect of base sequence on the DNA cross-linking properties of pyrrolobenzodiazepine (PBD) dimers. Nucleic acid Res. 2011; 39(13): 5800-5812. doi: 10.1093/nar/gkr122
8. Kitson SL, Cuccurullo V, Ciarmiello A, Salvo D, Mansi L. Clinical applications of positron emission tomography (PET) imaging in medicine: oncology, brain diseases and cardiology. Curr Radiopharm. 2009; 2: 224-253. doi: 10.2174/1874471010902040224
9. Mansi L, Ciarmiello A, Cuccurullo V. PET/MRI and the revolution of the third eye. Eur J Nucl Med Mol Imaging. 2012; 39(10): 1519-1524. doi: 10.1007/s00259-012-2185-x
10. Winter G, Harris WJ. Humanized antibodies. Trends Pharmacol Sci. 1993; 14(5): 139-143. doi: 10.1016/0165-6147(93)90197-r
11. Vegt E, Jong de M, Wetzels JMF, et al. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J Nucl Med. 2010; 51(7): 1049-1058. doi: 10.2967/ jnumed.110.075101
12. Paganelli G, Bartolomei M, Ferrari M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001; 16(3): 227-235. doi: 10.1089/10849780152389410
13. Palm S, Elgqvist J, Jacobsson L. Patient-specific alphaparticle dosimetry. Curr Radiopharm. 2011; 4(4): 329-335. doi: 10.2174/1874471011104040329
14. Sgouros G, Hobbs RF, Song H. Modelling and dosimetry for alpha-particle therapy. Curr Radiopharm. 2011; 4(3): 261- 265. doi: 10.2174/1874471011104030261
15. Brans B, Linden O, Giammarile F, Tennvall J, Punt C. Clinical applications of newer radionuclide therapies. Eur J Cancer. 2006; 42(8): 994-1003. doi: http://dx.doi.org/10.1016/j. ejca.2005.12.020
16. Lindegren S, Frost SH. Pretargeted radioimmunotherapy with α-particle emitting radionuclides. Curr Radiopharm. 2011; 4(3): 248-260. doi: 10.2174/1874471011104030248
17. Miao Y, Hylarides M, Fisher DR, et al. Melanoma therapy via peptide-targeted (alpha)-radiation. Clin Cancer Res. 2005; 11(15): 5616-5621. doi: 10.1158/1078-0432.CCR-05-0619
18. Maecke HR, Reubi JC. Somatostatin receptors as targets for nuclear medicine imaging and radionuclide treatment. J Nucl Med. 2011; 52(6): 841-844. doi: 10.2967/jnumed.110.084236
19. Wang L, Tang K, Zhang Qi, et al. Somatostatin receptor-based molecular imaging and therapy for neuroendocrine tumors. BioMed Research International. 2013. doi: 10.2174/1568009053202054
20. Morgenstern A, Bruchertseifer F, Apostolidis C. Targeted alpha therapy with 213Bi. Curr Radiopharm. 2011; 4(4): 295-305. doi: 10.2174/1874471011104040295
21. Zalutsky MR, Vaidyanathan G. Astatine-211-labeled radiotherapeutics: an emerging approach to targeted alpha-particle radiotherapy. Curr Pharm Design. 2000; 6(14): 1433-1455. doi: 10.2174/1381612003399275
22. Scheinberg DA, McDevitt MR. Actinium-225 in targeted alpha-particle therapeutic applications. Curr Radiopharm. 2011; 4(4): 306-320. doi: 10.2174/1874471011104040306
23. Ogawa K, Washiyama K. Bone target radiotracers for palliative therapy of bone metastases. Curr Med Chem. 2012; 19(20): 3290-3300. doi: 10.2174/092986712801215865
24. Salvatori M, Indovina L, Mansi L. Targeted α-particle therapy: a clinical overview. Curr Radiopharm. 2008; 1(3): 251-253. doi: 10.2174/1874471010801030251
25. El-Amm J, Freeman A, Patel N, Aragon-Ching JB. BoneTargeted therapies in metastatic castration-resistant prostate cancer: Evolving Paradigms. Prostate Cancer. 2013. doi: 10.1155/2013/210686
26. Jadvar H, Quinn DI. Targeted α-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013; 38(12): 966- 971. doi: 10.1097/RLU.0000000000000290
27. Liepe K. Alpharadin, a 223Ra-based alpha-particle-emitting pharmaceutical for the treatment of bone metastases in patients with cancer. Curr Opin Investig Drugs. 2009; 10(12): 1346- 1358.
28. U.S. Food and Drug AdministrationPress Release. FDA approves new drug for advanced prostate cancer. Website: http:// www.fda.gov/newsevents/newsroom/pressannouncements/ ucm352363.htm 2013; Accessed March 15, 2014.
29. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium223 and survival in metastatic prostate cancer. N Engl J Med. 2013; 369(3): 213-223. doi: 10.1056/NEJMoa1213755
30. Sartor O, Maalouf BN, Hauck CR, Macklis RM. Targeted use of Alpha Particles: Current Status in Cancer Therapeutics. J Nucl Med Radiat Ther. 2012; 3: 136. doi: 10.4172/2155- 9619.1000136
31. Carroll V, Demoin DW, Hoffman TJ, Jurisson SS. Inorganic chemistry in nuclear imaging and radiotherapy: current and future directions. Radiochimica Acta. 2012; 100: 653-667. doi: 10.1524/ract.2012.1964
32. Fisher DR. Commercial availability of alpha-emitting radio nuclides for medicine. Curr Radiopharm. 2008; 1: 127-134. doi: 10.2174/1874471010801030127
33. Kassis AI, Adelstein SJ. Radiobiologic principles in radionuclide Therapy. J Nucl Med. 2005; 46(Suppl): 4S-12S.
34. Unak P. Targeted tumour radiotherapy. Brazilian Archives of Biology and Technology. 2002; 45: 97-110. doi: http://dx.doi. org/10.1590/S1516-89132002000500014
35. Regaud C, Lacassagne A. La radiosensibilite cellulaire envisage dans ses manifestations generalis. In- Radiophysiologie et radiotherapie, Paris. Archieves de L’Institut du Radium de L’Universite de Paris and La Foundation Curie. 1927; 95-116.
36. Dahle J, Abbas N, Bruland ØS, Larsen RH. Toxicity and relative biological effectiveness of alpha emitting radioimmumoconjugates. Curr Radiopharm. 2011; 4(4): 321-328. doi: 10.2174/1874471011104040321
37. Cascini GL, Cuccurullo V, Tamburrini O, Rotondo A, Mansi L. Peptide imaging with somatostatin analogues: more than cancer probes. Curr Radiopharm. 2013; 6(1): 36-40. doi: 10.2174/1874471011306010006
38. Cuccurullo V, Mansi L. Toward tailored medicine (and beyond): the phaeochromocytoma and paraganglioma model. Eur J Nucl Med Mol Imaging. 2012; 39(8): 1262-1265. doi: 10.1007/ s00259-012-2156-2
39. Jurcic GJ, Larson SM, Sgouros G, et al. Targeted alpha particle immunotherapy for myeloid leukaemia. Blood. 2002; 100(4): 1233-1239.
40. Andersson H, Cederkrantz E, Bäck T, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients; pharmacokinetics and dosimetry of 211At-MX35 F(ab’)2 -a phase I study. J Nucl Med. 2009; 50(7): 1153-1160.
41. Harrison MR, Wong TZ, Armstrong AJ, George DJ. Radium223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag Res. 2013; 5: 1-14. doi: 10.2147/CMAR.S25537
42. de Bruyn M, Rybczynska AA, Wei Y, et al. Melanoma-associated Chondroitin Sulfate Proteoglycan (MCSP)-targeted delivery of soluble TRAIL potently inhibits melanoma outgrowth in vitro and in vivo. Mol Cancer. 2010; 9: 301. doi: 10.1186/1476-4598-9-301
43. Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012; 366(21): 2008-2016. doi: 10.1056/ NEJMct1114348
44. Zalutsky MR, Reardon DA, Akabani G, et al. Clinical experience with α-particle-emitting 211 At: treatment of recurrent brain tumor patients with 211At-labeled chimeric antitenascin monoclonal antibody 81C6. J Nucl Med. 2008; 49(1): 30-38. doi: 10.2967/jnumed.107.046938
45. Wang L, Chen H, Pourgholami MH, Beretov J, Hao J, et al. (2011) Anti-MUC1 monoclonal antibody (C595) and doce taxel markedly reduce tumor burden and ascites, and prolong survival in an in vivo Ovarian Cancer Model. PLoS ONE. 6(9): e24405. doi: 10.1371/journal.pone.0024405
46. Allen BJ, Tian Z, Rizvi SMA, Li Y, Ranson M. Preclinical stiudies of targeted alpha therapy for breast cancer using 213Bilabelled-plasminogen activator inhibitor type 2. Brit J Cancer. 2003; 88(6): 944-950. doi: 10.1038/sj.bjc.6600838
47. Smith-Jones PM, Vallabhajosula, S, Navarro, V, Bastidas, D, Goldsmith, SJ, Bander NH. Radiolabeled monoclonal antibodies specific to the Extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP Human Prostate Tumor. J Nucl Med. 2003; 44(4): 610-617.
48. Cohen MH, Shen Y Li, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009; 14(11): 1131- 1138. doi: 10.1634/theoncologist.2009-0121
49. Mayes S, Brown N, Illidge TM. New antibody drug treatments for lymphoma. Expert Opin Biol Ther. 2011; 11(5): 623- 640. doi: 10.1517/14712598.2011.560569
50. Lin FI, Iagaru A. Current concepts and future directions in radioimmunotherapy. Curr Drug Discov Technol. 2010; 7(4): 253-262. doi: 10.2174/157016310793360684
51. Kitson SL, Cuccurullo V, Moody TS, Mansi L. Radionuclide antibody-conjugates, a targeted therapy towards cancer. Curr Radiopharm. 2013; 6(2): 57-71. doi: 10.2174/1874471011306020001
52. Otte A. Diagnostic imaging prior to 90Y-ibritumomab tiuxetan (Zevalin) treatment in follicular non-Hodgkin’s lymphoma. Hell J Nucl Med. 2008; 11(1): 12-15.
53. Cicone F, Baldini R, Cox MC, et al. Radioimmunotherapy of heavily pre-treated, non-Hodgkin’s lymphoma patients: efficacy and safety in a routine setting. Anticancer Res. 2009; 29(11): 4771-4778.
54. Mace JR. Radioimmunotherapy in follicular lymphoma: an update. Clin Ad. Hematol Oncol. 2012; 10(6): 394-396.
55. Teicher BA, Chari RVJ. Antibody conjugate therapeutics: challenges and potential. Clin Cancer Res. 2011; 17(20): 6389- 6397. doi: 10.1158/1078-0432.CCR-11-1417
56. U.S. Food and Drug AdministrationPress Release. FDA approves Adcetris to treat two types of lymphoma. Website: http:// www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ ucm268781.htm 2011; Accessed March 15, 2014.
57. FDA approves new treatment for late-stage breast cancer: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm340704.htm 2013; Accessed March 15, 2014.
58. Bhatt S, Ashlock BM, Natkunam Y, et al. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood. 2013; 122(7): 1233-1242. doi: 10.1182/blood-2013-01-481713
59. Senter PD, Sievers EL. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat Biotechnol. 2012; 30(7): 631-637. doi: 10.1038/nbt.2289
60. Nolting B. Linker technologies for antibody-drug conjugates. Methods Mol Biol. 2013; 1045: 71-100. doi: 10.1007/978-1- 62703-541-5_5
61. Beck A, Lambert J, Sun M, Lin K. Fourth world antibodydrug conjugate summit: February 29-March 1, 2012, Frankfurt, Germany. MAbs. 2012; 4(6): 637-647. doi: 10.4161/ mabs.21697
62. Dosio F, Brusa P, Cattel L. Immunotoxins and anticancer drug conjugate assemblies: The role of the linkage between components.Toxins (Basel). 2011; 3(7): 848 883. doi: 10.3390/toxins3070848
63. Ducry L, Stump B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjugate Chem. 2010; 21(1): 5-13. doi: 10.1021/bc9002019
64. Doronina SO, Toki BE, Torgov MY, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol. 2003; 21(7): 778-784. doi: 10.1038/ nbt832
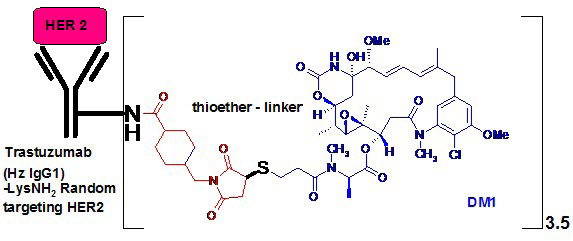
65. LoRusso PM, Weiss D, Guardino E, Girish S, Sliwkowski MX. Trastuzumab emtansine: a unique antibody-drug conjugate in development for human epidermal growth factor receptor 2-positive cancer. Clin Cancer Res. 2011; 17(20): 6437-6447. doi: 10.1158/1078-0432.CCR-11-0762
66. Kozak KR, Tsai SP, Fourie-O’Donohue A, et al. Total antibody quantification for MMAE-conjugated antibody-drug conjugates: impact of assay format and reagents. Bioconjug Chem. 2013; 24(5): 772-779. doi: 10.1021/bc300491k
67. Tijink BM, Buter J, De Bree R, et al. A Phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus. Clin Cancer Res. 2006; 12(20): 6064-6072. doi: 10.1158/1078-0432.CCR-06-0910
68. Sassoon I, Blanc V. Antibody-drug conjugate (ADC) clinical pipeline review. Methods Mol Biol. 2013; 1045: 1-27. doi: 10.1007/978-1-62703-541-5_1
69. Ouyang J. Drug-to-antibody ratio (DAR) and drug load distribution by hydrophobic interaction chromatography and reversed phase high-performance liquid chromatography. Methods Mol Biol. 2013; 1045: 275-283. doi: 10.1007/978-1-62703- 541-5_17