INTRODUCTION
This case outlines a 74-year-old male with ischemic cardiomyopathy with a left ventricular ejection fraction of 35% with history of cardiac resynchronization therapy-defibrillator (CRT-D) implantation. He presented with a new drop in functional New York Heart Association (NYHA) heart failure classification, which continued to worsen despite attempts at coronary revascularization and initiation of milrinone therapy. Later hospitalization revealed a change in QRS morphology. Further device interrogation revealed that the patient was losing left ventricle (LV) capture with deep inspiration in a supine position. After replacement of the patient’s LV lead, the patient had significant improvement in NYHA functional status, and was transitioned to oral heart failure medications.
CASE REPORT
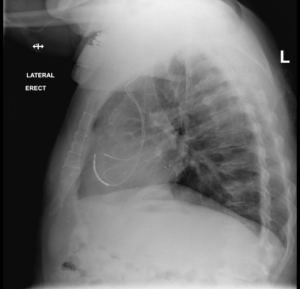
A 74-year-old male with a past medical history significant for coronary artery disease status post coronary artery bypass graft (CABG) in the late 1990s, ischemic cardiomyopathy with an ejection fraction of 35%. A CRT-D Model 3231 (St. Jude Medical, Little Canada, Minnesota, USA) was placed in 2011 at our institution consisting of a right atrial (model 1999), right ventricular (model 7-122Q), and a left ventricular bipolar lead (model 1156T) placed in a posterior-lateral position (Figure 1). Optimal programming and delays were unchanged since implant and provided 94% to 96% biventricular (Bi-V) pacing, and the patient was noted to have a 4-6% PVC burden. Of note, the lead capture thresholds for the LV lead remained consistent between 1.75-2.25 V @ 1.5 ms at all subsequent device checks post-implant. The LV output was programmed at 3.5V and remained thru the duration of follow-up.
Figure 1. Lateral Chest X-ray Revealing Biventricular Device Lead Placement
The most recent echocardiogram prior to his visit the clinic revealed an ejection fraction of 35%, moderate mitral regurgitation, and grossly normal appearing aortic, pulmonic and tricuspid valves. There was no evidence of elevated pulmonary artery systolic pressures. Nuclear viability testing with thalium-201 revealed a large area of inferior infarction with evidence of viability in the inferolateral wall.
The patient presented 1/2017 to the cardiology clinic with significant symptoms of dyspnea on exertion. His NYHA functional class dropped from class I to IV. Due to his symptoms over the course of the year he underwent multiple testing including left heart catheterization, device interrogation, imaging and extensive lab work. Lab work showed normal hemoglobin, chronic kidney disease (CKD) stage III with a baseline creatinine of 1.5, and an elevated brain naturietic peptide (BNP).
His initial left heart catheterization revealed multiple obstructive lesions that were thought to be contributing to his symptoms. The patient underwent percutaneous intervention to his large first obtuse marginal coronary artery (OM 1) branch after instantaneous flow reserve (IFR) testing confirmed significance of this lesion. Due to refractory symptoms approximately 3-months post-procedure, the patient was taken back to the catheterization lab to attempt revascularization of the left circumflex chronic total occlusion which was unsuccessful. He did undergo percutaneous coronary intervention (PCI) to his proximal left anterior descending coronary artery (LAD) as there were significant left to right collaterals supplying the right posterior descending artery.
Despite multiple coronary interventions, he continued to have a drop in his NYHA functional class. Given his reduced ejection fraction, functional status, and appropriate functioning of his CRT-D, he eventually underwent right heart catheterization which revealed a cardiac index of 1.49 and a cardiac output of 3.27 based on Fick calculation. At this point the patient was initiated on milrinone and had significant improvement in his NYHA functional class, from IV to I.
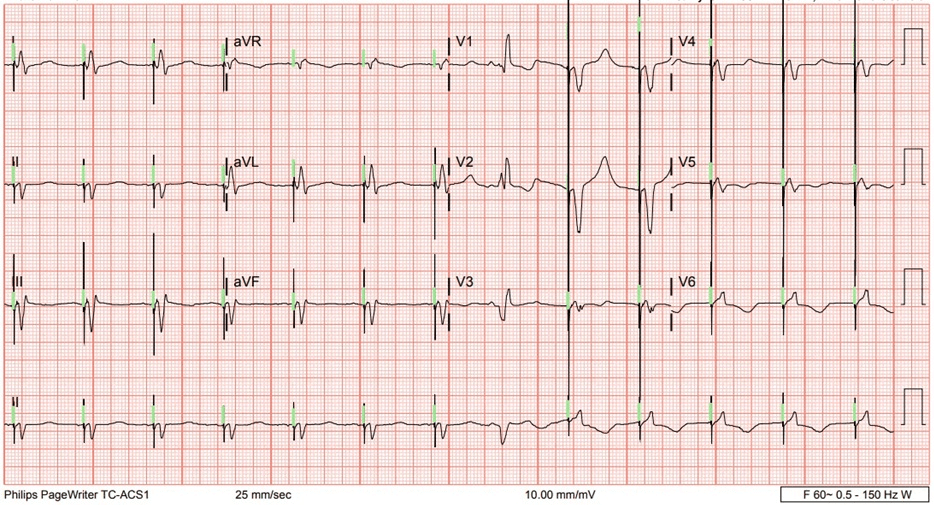
We initiated evaluation for advanced heart failure therapies, given his response to milrinone and he was classified as intermacs.3 Due to his age he was being evaluated for destination therapy left ventricular assist device. During this process he had another non-heart failure related admission where telemetry revealed sparse changes in QRS morphology which led to an 12 lead electrocardiogram (ECG) capturing the phenomenon (Figure 2). Re-interrogation of the device showed 94% Bi-V pacing as before and a consistent LV capture threshold of 2.25 Volts @ 1.5 ms in an right ventricle (RV) ring to RV coil configuration which was the programmed vector, as well as a 4.5 Volts @ 1.5 ms in the RV tip to configurations. Upon having the patient perform isometric movements no ECG changes were noted. Upon deep inspiration in the supine position, it was noted on telemetry that there was a distinct morphology change. At that time, an LV only pacing was initiated in a temporary mode and it was revealed that capture was lost upon inspiration in a supine position even with the output at 6 Volts. The patient subsequently underwent a LV lead revision and his original bipolar lead placed from 2011 was removed and was upgraded to a model Quartet 1458Q (St. Jude Medical, Little Canada, Minnesota, USA) quadrapolar lead successfully placed into the same branch and position and programmed to an M3-M2 pacing vector. A new model 3369-40 pulse generator was utilized and capture assessed in all positions including supine and with deep breathing and demonstrated no intermittent loss of capture, assuring CRT therapy on a more consistent basis and accurate percentages reported by the device. Eventually the patient was weaned off of milrinone successfully, and remains on oral heart failure therapies with resolution in a majority of his symptoms (Table 1).
Figure 2. 12-Lead ECG Demonstrating QRS Morphology Change
| Table 1. Hematology and Serum Chemistries Drawn in Working up the Patient’s Shortness of Breath |
| Hematology |
| WBC |
5.2 k/mm3 |
| BC |
4.29 M/mm3 |
| Hgb |
11.6 gm/dL |
| Hct |
34.4% |
| MCV |
80.3 fl |
| MCH |
27.0 pg |
| MCHC |
33.6 g/dL |
| RDW |
14.8% |
| Plt Count |
271 K/mm3 |
| MPV |
7.0 fl |
| Neut% |
68.3% |
| Lymph % |
18.3% |
| Mono % |
8.2% |
| Eos % |
0.2 K/mm3 |
| Baso% |
0.1K/mm3 |
| Chemistry |
| Sodium |
141 mmol/L |
| Potassium |
4.1 mmol/L |
| Chloride |
104 mmol/L |
| CarbonDioxide |
30 mmol/L |
| AnionGap |
7 |
| BUN |
30 mg/dL |
| Creatinine |
1.5 mg/dL |
| EstimatedGFR |
45 |
| Glucose |
151 |
| Hemoglobin A1C |
5.4% |
| Lactate |
0.9 mmol/L |
| Magnesium |
2.1 mg/dL |
| Total Creatine Kinase |
138 IU/L |
| Troponin I |
0.02 ng/mL |
| B-Natriuretic Peptide |
176 pg/mL |
| TSH |
2.90 uIU/mL |
DISCUSSION
Congestive heart failure (CHF) is the one of the most common discharge diagnosis in the United States. Approximately 5.7 million adults in the United States currently have CHF of which 55 percent have heart failure with preserved ejection and the other 55 percent have heart failure with reduced ejection fraction.1 There is significant morbidity, mortality, and cost-associated with diagnosis of heart failure. CRT along with guideline directed medical therapy has become the cornerstone of treatment of heart failure and has reduced the incidence of adverse outcomes. CRT is known to reduce risk of death and heart failure events in patients with reduced ejection fraction and wide QRS compared to use of implantable cardiac defibrillator (ICD) alone.2 Unfortunately, despite these advances, approximately 5% of all heart failure patients progress to end stage heart failure or advanced heart failure. The term advanced heart failure or end stage heart failure encompasses all patients who remain symptomatic and have refractory heart failure despite maximally tolerated pharmacotherapy and CRT implantation. Currently, the estimated number of American patients affected by advanced heart failure is 5.8 million.3 Once the patient is determined to have advanced heart failure, treatment options include heart transplantation or left ventricular assist device depending on comorbidities and age.
Our patient enjoyed NYHA class one symptoms for many years due to CRT and guideline directed medical therapy (GDMT). Once he started having symptoms, his device interrogation consistently showed a pacing percentage of 94-96% and adequate pacing margins. However, given his refractory symptoms, drop in ejection fraction over time and ECG in Figure 1, we hypothesized this patient may have had a substantial reduction in effective biventricular pacing. We confirmed this as the patient lost LV capture with ambulation on telemetry and deep inspiration. This resulted as the lead would reposition and mimic right ventricular pacing. Given his symptoms prior to and after repositioning the lead the burden of ineffective pacing was substantial and also correlated with a drop in his ejection fraction. Hays et al4 reported in a >30,000 patient analysis from the aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE) study that both symptoms and event-driven outcomes are continuously influenced by the percentage of biventricular pacing. In their analysis, in addition to improvement in symptoms, increased percentages of pacing was associated with a significant reduction in mortality.
Right ventricular pacing is known to induce electrical and mechanical dyssynchrony and can precipitate advanced heart failure if left untreated resulting in increased mortality. In an article by Kiehl et al5 found that pacemaker induced cardiomyopathy had an incidence of approximately 12.3% in patients with preserved pre-implant ejection fractions. They also reported that a right ventricular pacing burden over 20% was strongly associated with development of pacemaker induced cardiomyopathy. Furthermore, the BLOCK-HF6 trial studied patients with pre-existing congestive heart failure with high degree heart block and randomized them to receive either dual chamber device or a biventricular device. After 3-years of follow-up the biventricular pacing group had a 7% absolute risk reduction in death, urgent heart failure care and adverse LV remodeling compared with right ventricular pacing. In this case, although the percent of biventricular pacing reported by the device seemed acceptable, the effective Bi-V pacing was not adequate and this patient developed right ventricle pacing cardiomyopathy which eventually precipitated advanced heart failure and the patient became intermacs 3 on milrinone.
Although calculating the percentage loss of LV capture which may have inadvertently caused right ventricular only pacing, this phenomenon may account for some non-response seen in clinical practice which has been shown to range from 25-30%. Our patient who already had a reduced ejection fraction perhaps could not tolerate the frequent right ventricular pacing induced dyssynchrony. The above case details the importance of addressing every therapeutic option for advanced heart failure patients and optimizing them fully. Recognizing left ventricular lead displacement may be difficult, but can ultimately make the difference in outcomes as outlined above in this patient. Ultimately, the patient’s outcome was favorable and they remain on optimal medical therapy with a drastic reduction in heart failure symptoms since our intervention on the CRT-D system, with few heart failure admissions.
To our knowledge this is the first reported case of advanced heart failure precipitated by left ventricular lead positioning resulting in ineffective pacing and worsening cardiomyopathy. Our case also highlights the importance of optimization of CRT prior to proceeding with advanced heart failure therapies for refractory heart failure symptoms. Ultimately the patient’s outcome was favorable and they remain on optimal medical therapy with a drastic reduction in heart failure symptoms.
CONCLUSION
Given the patient’s QRS morphology change demonstrated on hospital telemetry, Holter monitoring is a reasonable consideration. This may have shown the same QRS morphology change (widening of the QRS consistent with RV apical pacing), suggesting the possible need for LV lead revision. Additionally, with the emergence of multi-point LV pacing and device programming should account for patient positional changes. CRT devices remain an important intervention in advanced heart failure practice, and optimal settings are certainly key to their successful therapy.
CONSENT
The authors have received written informed consent from the
patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








