INTRODUCTION
Kidney transplantation is the best strategy for improving survival and quality of life in patients with end-stage renal disease. Living donor nephrectomy is a method that can reduce the waiting time for kidney transplantation and avoid dialysis in extreme cases. Unlike traditional procedures, it is a special surgery because it is performed on healthy patients. Therefore, the safety and efficiency of the process are extremely important.1 Reducing the incidence of complications, shortening hospital stays, accelerating recovery, and returning to normal work activities are the keys to increasing patient turnover.2 Since Ratner and his team performed the first laparoscopic living donor nephrectomy (LLDN) in 1995, this minimally invasive procedure has become the standard procedure for organ transplants. It has replaced traditional open donor nephrectomy (ODN) in many centers.3 Compared to ODN, LLDN has features such as lower cost, faster recovery, lower blood loss, and better cosmetic results. The aim of this study is to present the preliminary procedures of 120 laparoscopic live nephrectomies performed in one location.4
MATERIAL AND METHODS
A prospective study of people included all patients who underwent laparoscopic kidney donation.5 The patients were found suitable according to detailed criteria. During donor selection, the patient’s medical examination and images (e.g., chest x-ray, high-resolution computed tomography, abdominal ultrasound, and abdominal computed axial tomography) are taken. In addition, kidney function tests include estimated glomerular filtration rate (eGFR), 24-hour urinary creatinine clearance, kidney scintigraphy, and urinalysis.6
In addition, all potential donors underwent an electrocardiogram, ambulatory blood pressure monitoring, and echocardiography, as well as endoscopy, including gastroscopy and colonoscopy, if required.7 Diethylenetriamine pentaacetate (DTPA) is done routinely to determine the functional status of the kidney. The donor preoperative assessment requirements are listed in Table 1. Patients are consulted by a variety of specialists, like nephrologists, urologists, transplant doctors, and mental health care professionals.
| Table 1. Pre-operative Assessment for Laparoscopic Kidney Donation |
|
Laboratory test
|
CBC, RFT, LFT, Urinalysis, PT-INR |
| Pre-operative check-up |
2D ECHO
|
|
Imaging
|
Chest x-ray, HRCT, USG abdomen, CT abdomen, DTPA scan |
| Specialized medical consultations |
Nephrologist, Ophthalmologist, Cardiologist
|
Computed tomography (CT) imaging is a necessary and mandatory test in planning lap donor nephrectomy.8 It not only helps in measuring the size and number of renal arteries but is also helpful in achieving high-resolution angiography or 3D computed tomography. In cases of renal failure, removal of the left kidney is better to reduce the risk of failure (due to the long venous-arterial anastomosis vessel). The patient lies on his right side with 45° of flexion. In the case of the removal of the right kidney, the patient is positioned similarly, but on the left.9
The surgery is performed using a transperitoneal approach. A Veress needle is used to obtain pneumoperitoneum. First, a 10 mm umbilical trocar was inserted, and a pneumoperitoneum was obtained under 15 cm H2 O pressure. Four additional trocars (two 5 mm and two 10 mm) were inserted under 10 mm laparoscopic optical control.10
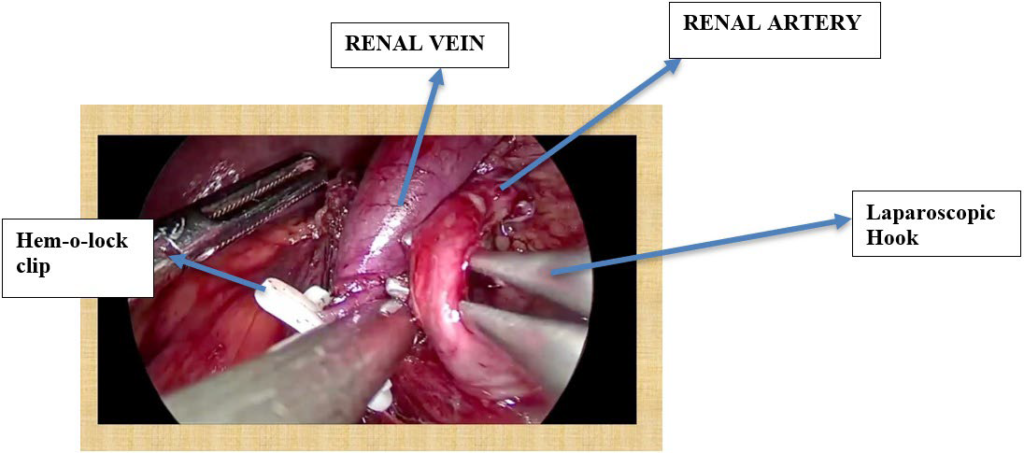
Ten (10) mm port inserted in the subcostal region. Another 10 mm was inserted in the midclavicular line. Five (5) mm in the epigastrium and another 5 mm in the anterior axillary line. Figures 1 and 2 show port placement for right and left donor nephrectomy, respectively. Figure 3 depicts hilar dissection.
Figure 1. Left Side Port Placement
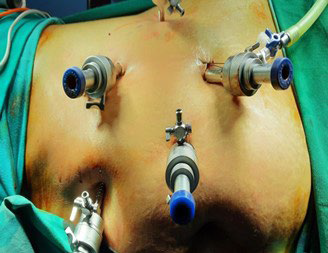
Figure 2. Right Side Port Placement
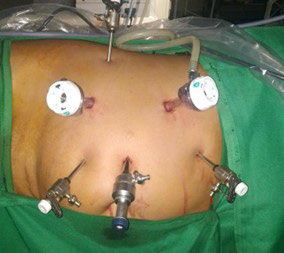
Figure 3. Hilar Dissection
Steps of Lap Donor Nephrectomy:
1. Port insertion
2. Colon mobilization
3. Ureter identification
4. Renal vein identification
5. Gonadal and adrenal vein clipping
6. Upper pole dissection and
7. Adrenal gland separation
8. Renal vein and renal artery skeletalization
9. Kidney mobilization from all around
10. Ureter clipping and cutting
11. Renal artery and renal vein clipping and cutting sequentially
12. Graft retrieval and perfusion.
13. The trocar is removed and the incision is closed.
RESULTS
A total of 120 laparoscopic living kidney surgeries were performed between 2018 and 2023. The mean age of the donors was 54 (range: 30-70), with 71 women and 49 men.
The average body mass index (BMI) is 24.5 kg/m2 (range: 19.4-33.3 kg/m2); the Eastern Cooperative Oncology Group (ECOG) performance average is 0; and the average Karnovsky activity is 100. Laboratory tests, including hemoglobin concentration, creatinine level, and eGFR, were calculated on days 7 and 30 using chronic kidney disease (CKD) formulae. We have performed a DTPA scan for 1 year.11 Patient demographics and laboratory results are shown in Table 2.
| Table 2. Demographics and Laboratory Results of Laparoscopic Kidney Donors |
|
Parameter
|
Value
|
| Age, mean (range), years |
54 (30-70) |
| Sex, women/men |
71/49 |
| Bmi, mean (range), kg/m2 |
24.5 (19.4-33.3) |
| Ecog performance status, mean |
0 |
| Karnofsky performance status, mean |
100 |
| Laboratory tests |
Pre-operative |
Post-operative (day 3) |
| Hemoglobin, mean, g/dL |
12.1 |
11 |
| Creatinine, mean, mg/dL |
0.99 |
1.25 |
| EGFR*, mean, ml/min/1.73 m2 |
82 |
55 |
The average operative time is 164 minutes and tends to be shorter in subsequent procedures. Of the removed organs, 109 were left kidneys, and 11 of them underwent right nephrectomy. All surgeries were performed laparoscopically without laparotomies. The mean warm-ischemia time was 4.83 minutes.
The average drainage time is 2.90-days. Of the graft vascular abnormalities, 34 and 12 patients had 2 and 3 renal arteries, respectively.11
Required vascular graft reconstruction was performed in seven cases due to abnormalities. The post-operative bleeding rate is nearly zero. The average blood loss is less than 100 mL. Intraoperative data are shown in Table 3.
| Table 3. Operative and Intraoperative Data for Laparoscopic Kidney Donation |
|
Parameter
|
Value
|
| Operation time, Mean±SD, Minutes |
164±28 |
| WIT*, Mean±SD, Minutes |
4.99±1.45 |
| Drainage time, Mean±SD, Days |
2.90±1.66 |
| Intraoperative blood loss, mean, mL |
84.4 |
| Left kidneys donated for transplantation (%) |
97.5 |
| Number of Renal Arteries (%) |
| 1 |
73 |
| 2 |
34 |
| 3 |
12 |
| Number of Renal Veins (%) |
| 1 |
115 |
| 2 |
5 |
Another intraoperative complication is lumbar venous bleeding, which is approximately 300 mL. The only post-operative problem is lymphatic leakage, which in the first case causes water to flow for a long time.12 There was no ureteral, intestinal, or mesenteric injury. The mean hospital stay was 3-days.
The donors were followed for a mean of 3.1-years (range: 0.5 to 5-years). The mean serum creatinine and concentration of eGFR at discharge and at final follow-up were 1.44 mg/dL and 1.22 mg/dL, respectively.12
No ureter, bowel, or mesentery injury Among the intraoperative complications, there is lumbar venous bleeding, which is approximately 300 mL. The only post-operative problem was lymphatic leakage, which was resolved in 2-weeks with conservative management. Each problem occurs in different patients and is not related to the progression of the learning curve for either operator. The mean hospital stay was 3-days.
The mean serum creatinine concentrations of donors at discharge and at the last follow-up were 1.44 mg/dL and 1.22 mg/ dL, respectively (Table 4).
| Table 4. Number of Surgeries by Year |
| 2018 |
16 |
| 2019 |
24 |
| 2020 |
10 |
| 2021 |
21 |
| 2022 |
36 |
| 2023 |
13 |
DISCUSSION
The LLDN procedure has become the standard procedure for kidney transplantation in the living population. It is characterized by less blood loss during surgery, fewer complications, faster recovery, and better cosmetic results. The above-mentioned advantages demonstrate the superiority of minimally invasive procedures over open surgery.
In addition to the classical open procedure (ODN), there are laparoscopic procedures for kidney transplantation: transperitoneal laparoscopy (LDN), manual transperitoneal laparoscopy (HALDN), retroperitoneoscopic (RDN), manual retroperitoneoscopic (HARDN), and robotically-assisted donor nephrectomy.
The surgical method used in the study at our center is transperitoneal laparoscopy, which is the result of the operator’s previous experience with this procedure. Our staff has extensive experience in laparoscopic kidney surgery and ensures that all 120 LLDN procedures are performed without complications.12 One patient was converted to open. For those unfamiliar with LLDN, the best option is a left nephrectomy with one artery and one kidney. A laparoscopic right kidney transplant is a more complex procedure.
Of the surgeries performed in our center, 109 were left kidney transplants and 11 were right kidney transplants. When selecting a kidney from a donor, we use the length of the artery as a guide. The patient’s record showed that the length of the left artery was the same or longer than that of the right artery in all cases.13
In LLDN surgery, the left kidney is preferred due to the lower risk of transplant failure. The warm ischemia time (WIT) of kidney transplant shows changes in the kidney, the time it takes from the clamping of the renal arteries to cooling the body with perfusate at a temperature of 4-6 °C. Body ischemia leads to a rapid loss of storage of high-energy products such as adenosine triphosphate (ATP) and the conversion of cellular metabolism to anaerobic metabolism. A combination of abnormal biochemical changes that cause physical damage, particularly in renal tubular epithelial cells, can lead to post-transplant acute renal failure. Organ transplants are at risk of graft failure if WIT persists.14
Therefore, all measures that can reduce WIT should be implemented during LLDN surgery. The optimal duration of WIT is in the range of 2 to 3-minutes. It has been reported in the literature that WIT for <0-minutes has no effect on delayed kidney function. In our patients, the mean WIT was 4.99-minutes, a value that did not affect the function of the graft.15 Intra-operative complications include injury to the hilar vessels that can cause blood loss of up to 300 mL, which can be successfully managed laparoscopically.
Without exception, all donated kidneys are transplanted to recipients. The 2001 classification of laparoscopic urological procedures by difficulty illustrates the problems of living kidney transplantation.16 The authors of the classification distinguish three factors (difficulty, operative risk, and anxiety), in which they classify radical nephrectomy as a difficult procedure (13 points out of 21) and live-patient nephrectomy as a very difficult operation. Difficult task (16 points out of 21).
The length of hospital stay (LOS) should be shorter because of the minimal effect of the LLDN procedure and the lower effect and shorter recovery time after illness. The average length of stay for our patients was 3-days. Although almost all donors are discharged early, some stay longer at our center and stay with transplant recipients. The ratio of kidneys collected from donors to organs from deceased donors has not changed much in recent years.
In recipients of laparoscopically procured grafts, we observed similar creatinine clearances 1-month after transplant in comparison to ODN. No significant differences in surgical complications, delayed function, acute and chronic rejection, or graft survival rates were found. Laparoscopic live donor nephrectomy has no adverse impact on recipient outcomes.17 The initial concerns about LDN and the recipient outcome were the longer operative duration and the pneumoperitoneum, resulting in problems with graft functioning. During early experience with LDN, increased warm ischemia time resulted in an increased proportion of delayed graft function. With increased experience, LDN and ODN have shown comparable graft function at short-term follow-up. One study evaluated factors related to potential delayed graft function after LDN. Identified variables included recipient age, human leukocyte antigen (HLA) mismatched donor recipient pairs, ischemia time, and transplantation of kidneys from a female donor into a male recipient.18 No variables associated with the laparoscopic technique itself were associated with poor graft function. In a randomized trial comparing ODN and LDN, graft function was no different between the groups as measured by the serum creatinine level.
Post-Operative Information
There is a decrease in operative time and an increase in laparoscopic nephrectomy observed. Complications were classified according to the modified Clavien classification (adapted from Koçak) used to evaluate complications in living kidney patients.19
The number of surgeries performed by year was 16 in 2018, 24 in 2019, 10 in 2020, 21 in 2021, 36 in 2022, and 13 in 2023. A total of 113 grafts were functional, and renal failure remained at normal levels at 12-month follow-up: 2 in 2019, 2 in 2020, 1 in 2021, and 2 in 2022.
CONCLUSION
Laparoscopic living kidney transplantation is a safe procedure and is the preferred method over laparotomy. LLDN is effective and successful with its short treatment time, low cost, and low mortality. Standardization of the surgical procedure increases the effectiveness and safety of the procedure and reduces the risk of complications for the donor.20 The learning curve in LLDN is longer, and in the early stages, surgical procedures will be difficult and the operative time will be longer. With the accumulation of experience in this field, the working time has also been shortened, comparable to the short time described in the literature.
Overall, the review of the literature shows that an LDN provides less post-operative pain, shorter hospital stays, a shorter period of rehabilitation, and an earlier return to normal work and physical activities in comparison to a conventional open flank nephrectomy. The complication rate is generally lower at our center, as we are accustomed to performing LDNs; however, complications can be lifethreatening and could impose significant costs on the health system. The risk-benefit assessment for choosing one procedure over another should be done meticulously. Laparoscopic live donor nephrectomy can now be performed with low morbidity and mortality for both donors and recipients and is proving to be the preferred operation for living donation. As with other minimally invasive procedures, patient demand has dictated widespread interest in the operation. This may increase the willingness to donate or to identify potential donors, ultimately resulting in the expansion of the organ pool. Finally, at any transplant center, the cost of the laparoscopic procedure should be considered.
The encouraging results of our first series on the use of minimally invasive procedures should have a positive impact on kidney donation, which is still insufficient compared to the number of patients awaiting names.
AUTHOR CONTRIBUTION
SJS ,TS and KNV are part of our transplant team doing donor and recipient part of the kidney transplantation. They have reviewed and edited the manuscript and given valuable inputs. AJ and CD collected and analysed the data.
FUNDING
None.
CONSENT
The authors have received written informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








