INTRODUCTION
Chronic subdural haematoma (CSDH) is one of the commonest forms of intracranial haemorrhage. Surgical drainage of CSDH is a routine operation in the modern neurosurgical practice.1,2 The incidence of CSDH is 8-58 per 100,000 in individuals over 65 years of age.3 However, with continuous rise of life expectancy together with a widening usage of anti-coagulants and anti-platelets medications worldwide, the incidence of this is likely to continue rising.4 Clinically, as the name suggests, CSDH does not present acutely and it may remain silent for variable periods of times and may present insidiously or with non-specific features. Surgical intervention has been shown to be the most effective way in treating this entity; however, the incidence of recurrence ranges from 9.2-26.5%.1,2,5,6,7 The recurrence can also remain silent with delay in diagnosis and associated morbidity and mortality.
We have conducted this Systematic review to evaluate the available literature addressing the risk factors for CSDH recurrence, aiming to minimise or at least identify patients at higher risk of recurrence in order to decrease associated morbidity. Moreover, this Systematic review will address areas where further research is required to provide robust evidence in the topic, as the implementation of evidence based medicine provides high quality standard medical care at the lowest cost.8
A brief analysis of the literature will be conducted using the Critical Appraisal Skills Programme (CASP) tool9 and then each study will be ranked using the Harbour and Miller10 hierarchy of ranking. See appendix I.
Search Strategy
Table 1 below summarises the search strategy used for the literature search.
| Table 1: Search Strategy. |
| Keywords |
The following key words were set to be recognised within article title, abstract, and/or keywords:
Subdural Hematoma, chronic subdural haematoma, recurrence, risk factors |
| Search terms |
-Chronic subdural haematoma (OR) subdural haematoma
-Recurrence (AND) risk factors
-Chronic subdural haematoma (OR) subdural haematoma, (AND) recurrence (AND) risk factors |
| Limitations |
The search was limited to the following:
English Language.
Humans.
Between 2012 and current date. |
| Inclusion criteria |
The search included patients with chronic subdural haematoma (unilateral or bilateral, surgically or non-surgically managed)
The search also included systematic reviews, RCTs, cohort studies, and literature reviews. |
| Exclusion criteria |
The search excluded:
Solely pregnant and post-partum patients.
Neonates and paediatrics.
Case reports.
Descriptive reports. |
| Databases used |
Ovid SP (MedLine/Embase), PubMed, Google Scholar |
| Screening evidenced |
Following the search, studies titles and abstracts were screened for relevance, inclusion and
exclusion criteria, and non-qualifying articles were then excluded. Reference lists of included papers were reviewed with ‘backward chaining’ employed to gather pertinent papers for consideration. |
| Final number |
6 studies will be addressed |
Ovid via Medline, PubMed and google scholar search resulted in 33 publications, which was then limited to studies in English language, Humans and the duration between 2012 and current date this limited the publications to 18 papers.
Following is a justification for the used search limitation:
English Language is an international language for healthcare, the majority of the top journals with high impact factors are in English, and is the most widely learned second language. Nevertheless, we are aware that by limiting the search to publications in a single language this could potentially affect the generalisability and possibly results in English language, selection, publication, and citation biases.11 The search was also limited to humans, given the limited role for the animal derived data in this topic.
With regards to publication period this was limited to the last 5 years to ensure contemporaneous evidence. Nevertheless, following the search, studies titles and abstracts were screened for relevance, and reference lists of included papers were reviewed with ‘backward chaining’ employed to include seminal papers. Following limiting the search to the above, 18 studies were screened, and limited by the type of this review only 6 papers will be discussed.
Review of Literature
CSDH is one of the most commonly encountered conditions in neurosurgery; however, there is no consensus regarding clinical features, correlating factors, or causes of recurrence.12 Clinically, recurrent bleed can also be challenging and both clinical and imaging factors can be used to make a positive diagnosis. Moreover, presence of a rebleed does not always result in repeat surgery and similarly, significant rebleed may remain clinically silent and undiagnosed for variable periods of time, potentially with adverse outcomes. It is therefore important that the risk factors associated with rebleeding are identified and such patients are observed and followed-up more closely. Multiple studies have been conducted to identify potential risk factors contributing to the pathogenesis of CSDH and its recurrence with numerous factors reported.12,13,14,15,16,17,18,19,20,21,22
Yamamoto et al13 attempted to determine independent predictors contributing to the recurrence of chronic subdural hematoma (CSDH) in 105 patients who underwent CSDH surgery over 9 years period, with follow-up computed tomography (CT) scanning performed 1 day, 1 week, 1 month, 3 months, and 6 months post-operatively. The criteria used to define recurrence were radiological; however, clinical recurrence (prompted by re appearance of symptoms) warranted earlier scanning. The radiological recurrence was an increase in the hematoma thickness and a change in hematoma density on follow-up CT scans within 3 months post-operatively.13 By using univariate and multivariate analyses to assess the relationships among various variables and CSDH recurrence Yamamoto et al13 reported four independent variables affect the recurrence of CSDH: a positive history of seizures and the width (maximum diameter) of the hematoma were positively associated with increased risk of recurrence, while a positive history of diabetes mellitus (DM) and the multiplicity of hematoma cavities (multiple membrane) on CT scans were both associated with less risk for recurrence. Brief discussion regarding these factors will follow.13 However, the aforementioned study was a retrospective cohort study, and thus is potentially subject to sources of bias and variation. The sample size of the study was limited, and when considering the incidence of the disease the study will be under-powered; hence, further investigation is required to assess the independent predictors revealed in the study. The study was therefore scored 2+ in the Harbour and Miller10 hierarchy of ranking.
Several studies support the role of seizure disorders, alcoholism, cerebrospinal fluid shunts, anticoagulation therapy and coagulopathies.13,23,24,25 These may be variably associated with head trauma, brain atrophy and decreased blood homeostasis. Seizures can be associated to the recurrence of CSDH due to the occasional head injury associated with certain types of seizures, or as a result of coagulopathy due to some anticonvulsants or to their effect on the liver causing disruption of the coagulation cascade.26
While the width of the hematoma is often determined at the level of the maximum thickness of the clot, it has been reported to be associated with the patient age, with the underlying atrophy of the aging brain providing the space for the hematoma to grow and/or recur.27 This may also lead to poor brain re-expansion after the operation. Poor brain re-expansion has been correlated with recurrence in previous reports.19,28
Hyperglycaemia secondary to diabetes mellitus is associated with vascular occlusive disorder secondary to the hyper-viscosity of the blood and the often encountered atherosclerosis.29 Yamamoto et al13 suggested that DM may play a role in decreasing the re-bleeding tendency of CSDH, since patients with DM has a high osmotic pressure and increased platelet aggregation.29 This theory could be supported by the findings of previous study which reported that osmotherapy performed using 20% mannitol is effective in stopping repeated bleeding of a CSDH.30 On the other hand capillary vasculopathy including haemorrhage (e.g., retinal haemorrhage) is one of the major complications in diabetic patients, and the exudation from the capillaries in the membrane of CSDH plays an important role in its enlargement.31,32 Moreover, similar study showed increased but non-significant risk for recurrence in patients with DM, it was also found that patients with bilateral CSDH tend to have DM.17
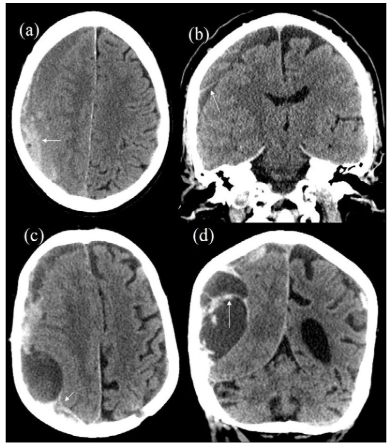
With regards to multiplicity of the hematoma cavity conflicting reports were published. While previous studies reported multiplicity to be positively correlated with recurrence of CSDH,2,33 other concluded it is associated with lower rates of recurrence.13 This conflicting evidence could be attributed at least partially to the discrepancy in defining “multiplicity of the haematoma”. In some studies with a positive correlation, the authors identified “multiplicity” as multiple CSDHs,2,33 whereas Yamamoto et al defined multiplicity of hematoma cavities as the involvement of multiple cavities, similar to what has been previously described as trabecular haematoma (Figure 1).5,13
Figure 1: CT Scans of 2 Different Patients (a,b) and (c,d) Show Multiplicity of Haematoma on Right Side with Blood Products of Different Attenuations along with Presence of a “Membrane” (White Arrow).
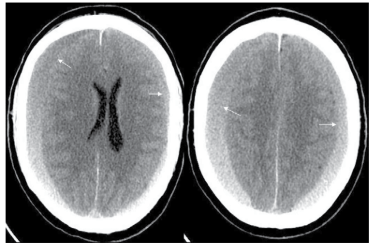
Torihashi et al17 conducted a study to determine independent predictors associated with CSDH recurrence. The results demonstrated that bilateral CSDH was an independent risk factor for the recurrence of CSDH. Although, anti-platelet and anticoagulant therapy had no statistically significant effect on CSDH recurrence, the time interval between the injury and the first operation for patients with anti-platelet and/or anti-coagulant therapy was shorter (29.9 vs. 44.2 days).17 The relative strengths of the above study were the bigger sample size and the fact they used a logistic regression model in performing a multivariate statistical analysis of the recurrence factors. Nonetheless being a retrospective study it scores 2++ in the Harbour and Miller hierarchy of ranking.10 Further studies also supported bilateral CSDH as a risk factor for recurrence (Figure 2).18,19 It is though that patients with bilateral CSDH tend to have previous brain atrophy increasing the risks of recurrence as discussed earlier.
Figure 2: CT Scan Showing Presence of Bilateral Subdural Haematomas (Arrows).
Abouzari et al20 conducted a study looking at the role of posture in post-operative patients in the recurrence of surgically managed traumatic CSDH.20 The study concluded that assuming an upright posture soon after burr-hole surgery is associated with an increased incidence of CSDH recurrence. Another study showed similar but statistically non-significant higher recurrence rate of CSDH with early sitting up posture in comparison to 3 days of bed rest.34 The limitations of Abouzari et al20 study was that they only studied patients with a history of head trauma and excluded those with shunts, seizures, alcohol abuse or use of anticoagulants. While up to 40% of patients with CSDH cannot recall a history of trauma,35 this very homogenous study group in the Abouzari et al20 trial brings the generalizability of the trial into question. In the same study recurrence was defined by radiological criteria, and despite the radiological recurrence rate was significantly higher in the patients who assumed a head-elevated position immediately after surgery, these recurrences did not seem to affect the patients’ clinical recovery and only one patient required surgery to drain the recurrent haematoma,20 the study was inadequately powered and no details for statistical analysis was included, therefore scored 1 in the Harbour and Miller hierarchy of ranking.10
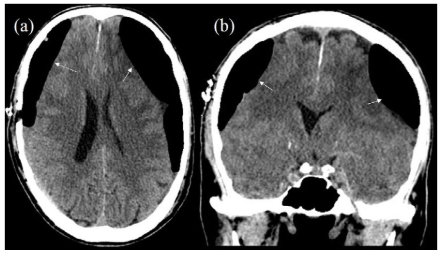
Another study looking at the “radiological factors” associated with risks of CSDH recurrence, showed increased risk of rebleed in patients with parietal or occipital drainage compared to those who had frontal burr hole drainage. It also showed that patient with residual subdural air on CT scans obtained 7 days post-surgery had a higher recurrence rate than those with no subdural air on the CT scan (Figure 3).12 Similar observation was drawn by Nagata et al36 showing that the amount of subdural air found postoperatively correlated negatively with the resolution rate of CSDH.
Figure 3: CT Scans Showing Significant Amount of Air in the Subdural Cavities on both Sides Post Drainage (White Arrows).
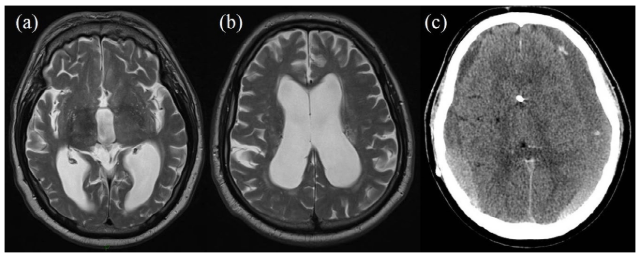
To further explain the effect of different risk factors, different theories have been proposed to explain post-operative recurrence of CSDH. One is the pressure difference theory which emphasises pressure imbalance between the outside and inside of the inner haematoma membrane (subdural space and the subarachnoid/subpial space); that is high pressure in the hematoma cavity and/or low pressure in the subarachnoid space (Figure 4). The earlier situation is indicated by massive subdural air collection, residual SDH and persistent widening of the hematoma cavity (ongoing bleeding in the subdural space). The latter situation is indicated by excessive fluid loss such as dehydration, anemia, excessive cerebrospinal fluid drainage or impact of severe brain atrophy.35,37 Moreover, Nakaguchi et al12 also reported that patients with a subdural space more than 10 mm wide on CT scans obtained 7 days post-surgery had a higher recurrence rate than those with a space measuring 10 mm or less. The study concluded that post-operative re-accumulation of CSDH can be reduced by placing the tip of the drainage catheter in the frontal convexity and by removing subdural air during or after surgery.12 This is explainable by the fact that air accumulates in the frontal convexity while the patient is supine immediately after surgery. With the same principle in mind for draining extra fluid and air from the subdural space, Cambridge conducted a randomised trial of using a subdural drain versus no drain following evacuation of CSDH concluded that the use of a drain after burr-hole drainage of chronic subdural haematoma is safe and associated with reduced recurrence and mortality at 6 months.38 Nakaguchi et al scored 2+ in the in the Harbour and Miller hierarchy of ranking, while the Cambridge trial scores 1+ as a well conducted randomised controlled trial with low risk of bias.10,38
Figure 4: MRI Scan (a, b) Shows Dilated Ventricular System in a Patient Clinically Presenting with Normal Pressure Hydrocephalus. Post Drainage, CT (c) Shows Bilateral Subdural Haematomas with a Decompressed Ventricular System.
Another theory is the inflammatory theory, first proposed In 1857 by Virchow who described CSDH as a dural inflammatory disease and called it “pachymeningitis hemorrhagica interna.” He was the first to stress the importance of inflammation for the onset and development of CSDH.39 Later on several studies demonstrated that CSDH is the result of a local inflammatory reaction of the dura to an injurious stimulus.35,40 Frati et al21 conducted a prospective study over 2 years period to determine role of local inflammation in the pathogenesis and postoperative recurrence of chronic subdural hematoma (CSDH).21 The study – although significantly under powered – has included only patients who can clearly recall history of head trauma, and showed evidence of an inflammatory process within the dural border cell layer, this has a clear impact on the generalisability of the trial, scoring 2+ in the Harbour and Miller hierarchy of ranking.10 The study concluded that higher levels of inflammatory cytokines were positively correlated with recurrence and re-accumulation of the CSDH. Frati et al advocated for a prolonged post-operative course of anti-inflammatory medicine given as prophylaxis to minimise the risks of CSDH recurrence. Similar rationale and conclusion were reached by another recently published study advocating the use of steroids following the surgical evacuation of CSDH to prevent recurrence.22 The role of steroids in CSDH remains a controversial topic; nonetheless, an ongoing trial in the UK is currently addressing this and hopefully will put an end to this debate.41
Most recently the British Neurosurgical Trainee Research Collaborative (BNTRC) published the largest multicenter, prospective, observational cohort study looking at the management and outcome for patients with chronic subdural haematomas.42 This has included centres throughout the United Kingdom (UK) and Ireland, and showed the rates of CSDH mortality (2%), symptomatic recurrence (9%), and unfavorable functional outcome (22%) were all acceptable when audited against predefined criteria from the literature.42 However, multivariate analysis demonstrated that failure to insert a drain intraoperatively independently predicted recurrence (p=0.011) as well as unfavorable functional outcome (p=0.048). Reinforcing previous studies conclusions, the BNTRC group detected statistically significant unfavorable functional outcomes following prescribed post-operative bed rest (p=0.019).20,35 It also concluded that Increasing patient age (p<0.00001) is associated with unfavorable functional outcome; however, there was no significant difference in relation to recurrence, consolidating previous reports recommendations.13
Unlike previous studies; the BNTRC had clear definition to the recurrence of CSDH, which was clinical recurrence of CSDH symptoms, confirmed radiologically, and requiring surgery within 60 days.42 On the other hand one of the study’s limitations was the lack of long term follow as patients were observed only during their admission course at the neurosurgical unit (NSU). Moreover, the study cohort was skewed to single surgical drainage technique (burr hole drainage) which was the modality used in 89% of operated cases; hence, making predicting outcome in patients treated with other surgical techniques (e.g., mini craniotomy) an area of ongoing debate.
The study nevertheless was well conducted and scores 2++ in the in the Harbour and Miller hierarchy of ranking (Table 2).
| Table 2: Summary of the Studies Discussed in this Paper, the Aim of Each, Concluded Factors for CSDH Recurrence, Strengths, Weaknesses, and Score in the Harbour and Miller hierarchy of ranking.10 |
| Article |
Aim of study |
Factors associated with
increased risk for CSDH
recurrence |
Strengths |
Weaknesses |
Score |
| Yamamoto et al2 |
To determine independent predictors contributing to the recurrence of CSDH |
– Width of the hematoma.
– Multiplicity of hematoma cavities.
– Seizures
– Negative history of DM |
– Clear definition for
recurrence
– Robust statistical analysis |
– Retrospective
– Small sample size |
2+ |
| Torihashi et al21 |
To determine independent predictors contributing to the recurrence of CSDH |
– Bilateral CSDH |
– Larger sample size
– Robust statistical analysis. |
– Retrospective |
2++ |
| Abouzari et al27 |
To evaluate the relationship between
recurrence rate of CSDH and patient posture postoperatively |
– Assuming an upright posture soon after burr-hole surgery |
– Randomized double linded
controlled trial |
-Generalizability
– Underpowered
– Radiologically defined recurrence with very limited clinical sequel |
1- |
| Nakaguchi et al8 |
To determine features of CSDHs
recurrence rate on the basis of the natural history of these lesions and their intracranial extension |
– Subdural space more than 10 mm wide on CT 7 days
post-surgery
– Subdural drain not placed on the frontal convexity
– Presence of subdural air, intra or post operatively
– Cranial base type of CSDHs was high |
– Prospective study
– Over 9 years
– Long term follow-up |
– Single center
– Small sample size
– Recurrence defined radiologically with no clinical correlation |
2+ |
| Frati et al34 |
To determine role of local inflammation in the pathogenesis and recurrence of CSDH |
– Higher levels of inflammatory cytokines |
– Prospective study |
– Under powered
– Generalizability |
2+ |
| Brennan et al42 |
To examine the management and outcome for patients with CSDH across the UK |
– Failure to insert a drain
intraoperatively |
– Multicenter
– Prospective
– Clear definition for
recurrence |
– Lack of long term follow-up
– Skewed to single surgical drainage technique |
2++ |
| CSDH: Chronic subdural haematoma; CT: Computed Tomography. |
CONCLUSION
The review highlights the lack of unified definition for CSDH recurrence as different studies use different methods in labelling recurrence; nonetheless the majority combine clinical features as well as imaging modalities to identify recurrence of CSDH. The available evidence is generally underpowered and more research is required in this topic.
There are different factors contributing to the recurrence of CSDH, which can be divided into patient factors, radiological factors, surgical/technical factors, and post-operative factors.
Patient factors include history of seizures, trauma, alcoholism, brain atrophy, and presence of CSF shunts, while there is conflicting evidence regarding the role of DM in relation to recurrence risk of CSDH.
Radiological factors include presence of air in the subdural space in the post-operative scan, width of the haematoma, width of the subdural space and presence of bilateral CSDH. The predictive value of presence of multiple membranes in the CSDH remains controversial.
With regards to the surgical factors, there are different techniques adopted, nonetheless it was found that burr hole craniotomy is the most adopted method, and there is lack of evidence testing outcomes of other surgical techniques. The risk of CSDH recurrence is higher in patients with parietal or occipital drainage compared to those who had frontal burr hole drainage. Placing a subdural drain was noted to decrease the chance of recurrence and some evidence showed better outcomes for frontally placed drains.
Post-operative patient positioning seems to affect the recurrence risk, with the current evidence promoting avoidance of early sitting up of patients with CSDH. It is clearly noted that more studies are necessary to address this topic.
The role of anti-inflammatory agents (including steroids) remains an area of hot debate. There is a need of well conducted adequately powered multicentre randomised trial(s) to increase our understanding and deliver more robust recommendation regarding the topic .
Finally, we have briefly described the factors thought to be associated with increased risk of recurrent CSDH and highlighted areas of ongoing debate.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interst.










