INTRODUCTION
Massive pulmonary gastric acid aspiration uniformly produces very severe, acute lung injury. At present there is no know interventional therapy for aspiration-induced acute lung injury. Emergent therapeutic approaches in the past have included careful volume limited mechanical ventilatory support along with appropriate critical care measures such as careful fluid management, vasopressor support, and broad spectrum antibiotics. Increasingly, extracorporeal membrane oxygenation (ECMO) is being employed to support patients following the onset of acute respiratory distress syndrome (ARDS) induced by a number of acute illnesses (e.g., sepsis, pneumonia, toxic inhalation, viral illnesses, etc). Here we describe the first use of high dosage intravenous vitamin C as an adjunctive measure to decrease the intensity of lung injury suffered in a patient following high volume gastric acid aspiration that rapidly led to ARDS.
CASE PRESENTATION
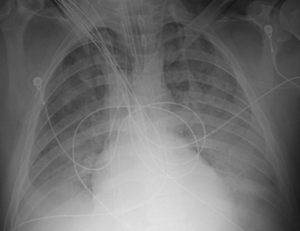
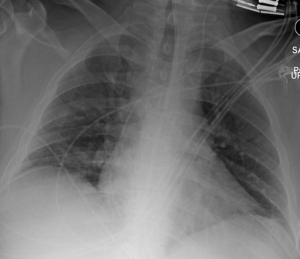
A 34-year-old male experienced a witnessed generalized tonic clonic seizure and gastric aspiration followed by 3 minutes of cardiopulmonary resuscitation for pulseless electrical activity arrest. The patient’s past medical history was significant for attention deficit/hyperactivity disorder, gastro-esophageal reflux disease, hypothyroidism and chronic lower back pain. There was no known history of seizure disorder. Postictal and post-return of spontaneous circulation, the patient was restless but following commands. The patient was normotensive. The cardiac exam revealed a regular tachycardia with no murmurs, gallops or rubs. No jugular venous distension was present. Chest exam revealed diffuse rhonchi bilaterally with diminished air movement. Blood was present in the mouth and nares. Upon arrival at an outside hospital, tracheal intubation and volume-limited mechanical ventilation was begun. Oxygenation failed to improve despite multiple attempts to optimize ventilatory support with a positive end expiratory pressure (PEEP) of 18 cm of water, FiO2 of 1.0, and inhaled nitric oxide (iNO) at 20 parts per million (ppm). The patient was subsequently transferred to Virginia Commonwealth University Medical Center for ECMO support. Upon arrival, assist-control, pressure-control ventilation was continued. Delivered PEEP, oxygen fraction and iNO were unchanged. Anterior-posterior chest X-ray imaging revealed diffuse bilateral opacification consistent with acute respiratory distress syndrome (Figure 1). Subsequent computed tomographic (CT) imaging of the brain was found to be within normal limits.
Figure 1: Hospital Day 1, Anterior-Posterior Chest x-ray Demonstrating Widespread Pulmonary Edema. ECMO Support Initiated.
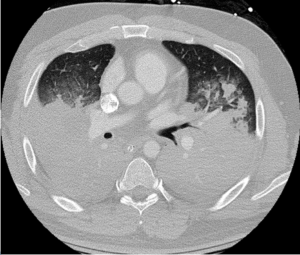
With ongoing hypoxemic respiratory failure in the setting of severe ARDS, veno-venous ECMO was instituted, employing a 31 French Avalon Elite® Bi-Caval Dual Lumen Catheter via the right internal jugular vein. Rotational frequency was set to maintain a flow of 5 liters per minute. Bilvalirudin was initiated for device anticoagulation, maintaining a partial thromboplastin time of 60-80 seconds. Hydromorphone and propofol infusions were administered for analgesia and sedation. Volume limited mechanical ventilation (tidal volume, 3-4 ml/kg) was continued. Vancomycin and pipercillin-tazobactam were selected as antibiotic therapy. CT angiography of the chest was negative for pulmonary emboli but revealed near-complete opacification of bilateral hemi-thoraces with modest preservation of aeration of the middle lobe, lingula and apical upper lobe segments (Figure 2). Bronchoscopy revealed the presence of extensive blood clots throughout the tracheobronchial tree with severe mucosal hyperemia. Active bleeding was not identified.
Figure 2: Hospital Day 1: Chest CT Imaging Demonstrating Widespread Consolidation of Lung Consistent with ARDS.
On hospital day 2 (ECMO day 2), high dose intravenous ascorbic acid was initiated at 50 mg/kg every 6 hours. Methylprednisolone (2 mg/kg every 6 hours) was initiated. Also this day, furosemide was infused to maintain a negative fluid balance. Repeat bronchoscopy was performed on ECMO day 3 which demonstrated decreased mucosal hyperemia, and the absence of active bleeding.
Over subsequent days, lung compliance, oxygenation and ventilation improved significantly as did chest imaging. Repeat non-contrast CT imaging of the chest on ECMO day 6 revealed significantly less opacification with improved lung aeration (Figure 3). A methylprednisolone taper was initiated. Hospital day 6, ECMO was successfully trialed on a sweep flow of 0 liters/minute. Ascorbic acid dosing was decreased to 25 mg/kg every 6 hours. A respiratory culture obtained this day revealed gram negative rods and gram positive cocci, ultimately speciated as Klebsiella pneumoniae and methicillin-resistant Staphylococcus aureus (MRSA). The patient was liberated from ECMO on hospital day 7. Chest imaging with anterior-posterior chest X-ray on the day ECMO was stopped continued to reveal substantial radiographic improvement (Figure 4).
Figure 3: Computed Tomographic Imaging of the Chest Hospital Day 6, ECMO Day 6 Reveals Significant Resolution of Pulmonary Edema and Airspace Opacities.
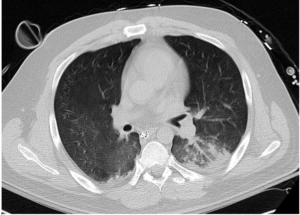
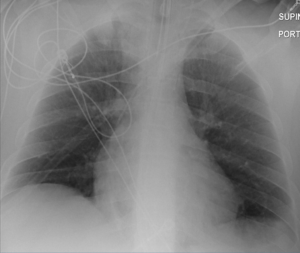
Figure 4: Anterior-Posterior Chest X-ray Hospital Day 7 Continues to Reveal Substantial Improvement.
On hospital day 8, ascorbic acid infusions were discontinued. Repeat respiratory cultures on hospital day 11 continued to demonstrate MRSA and ceftaroline was initiated. The patient was liberated from mechanical ventilation on hospital day 13 (Figure 5). He was discharged to home on hospital day 19.
Figure 5: Hospital Day 13 Mechanical Ventilation was Discontinued. AP Chest X-ray Following Extubation Reveals Continued Resolution of ARDS.
DISCUSSION
No effective therapy has previously been described for gastric acid-induced ARDS. High dosage intravenous vitamin C as described in this report and other reports from Virginia Commonwealth University Richmond, VA, USA, have proven to be effective adjunctive anti-inflammatory therapies. We report here the first application of high dose intravenous vitamin C employed as an interventional drug therapy for gastric acid-induced ARDS. The use of intravenous vitamin C to treat acute lung injury is still investigational and is the subject of an ongoing NIH-funded double-blinded placebo-controlled multicenter trial, examining its use in sepsis-induced ARDS. Few studies have reported the use of intravenous vitamin C in critically ill patients with ARDS. Sawyer et al2 reported that large intravenous doses of ascorbic acid plus other antioxidants (i.e., tocopherol, N-acetyl-cysteine, selenium), in patients with ARDS reduced mortality by 50%. Nathens and colleagues infused ascorbic acid intravenously at 1 gram every 8 hours co-administered with oral vitamin E for 28 days in 594 surgically critically ill patients.3 They found a lower incidence of multiple organ failure and acute lung injury. Tanaka and colleagues infused ascorbic acid at a dosage of 66 mg/kg/hour for the first 24 hours in patients suffering greater than 50% surface area burns and showed significantly reduced thermal injury-induced capillary permeability with significantly lower intravenous volume required for resuscitation.4 Increasing plasma ascorbic acid levels in hospitalized patients following gastric acid-induced ARDS is not part of current critical care practice. Vitamin C dosages utilized in the treatment of the patient we describe in this case report of gastric acid-induced ARDS arose from our previous human studies where we infused high doses intravenous vitamin C into critically ill patients with severe sepsis5 and in our preclinical animal studies.6,7,8 Our preclinical studies clearly show that vitamin C exerts robust “pleiotropic effects” when utilized as reported in the care of this patient with gastric acid-induced ARDS. In a recent study in critically ill patients with severe sepsis, we infused vitamin C at 50 mg/kg every 6 hours and found a marked reduction in multiple organ injury with reduced inflammatory biomarker levels in plasma.5 Our preclinical work in septic, lung-injured animals, shows that vitamin C down-regulates pro-inflammatory genes driven by transcription factor NF-κB.7 Early work by Kennedy et al and Goldman et al previously established that pulmonary gastric acid aspiration promotes an “acid burn” in lung, leading to rapid loss of lung barrier function as activated neutrophils migrate into the airspaces, producing tissue injury by liberating reactive oxygen species and proteolytic enzymes.9,10 As a consequence, following acid aspiration, pulmonary edema forms rapidly as we report here with the acute onset of respiratory failure. Our prior research with vitamin C has revealed that parenteral infusion of vitamin C significantly increases alveolar fluid clearance in septic lung-injured animals.8 Further, our research shows that vitamin C down-regulates neutrophil extracellular trap formation, a newly described phenomenon where circulating blood neutrophils exposed to certain pro-inflammatory stimuli disgorge their genomic DNA into their peri-cellular space.11 Extracellular DNA from the neutrophil contains adherent lysosomal enzymes which remain active and capable of damaging lung microcirculation, thus leading to pulmonary capillary damage.12 Infused vitamin C is also capable of dismuting liberated reactive oxygen and nitrogen species which also appears necessary for attenuating lung injury.13
Prior reports have described ARDS with such severity that ECMO was required. Wetsch et al described a 39-year-old man who sustained massive gastric acid aspiration with resultant ARDS who was supported with ECMO.14 During the hospital course of the patient we report here, short-term methyl prednisolone was also infused and likely played an undetermined role in promoting resolution of the patient’s lung injury. Zhao et al15 recently reported a retrospective analysis of 73 acute stroke patients who were diagnosed with aspiration-related ARDS and in whom the hospital mortality rate was 39.7%. Short-term corticosteroids reported in Zhao’s study were administered in 47 patients (64.4 %) at a mean dosage of 1.14 mg/kg per day of methylprednisolone via intravenous infusion for a period of 7.3 days. Ground glass opacities reported in chest computed tomography images in Zhao’s study improved when corticosteroids were administered. However, none of the ARDS patients Zhao reported sustained the extent of lung injury similar to the patient we describe here and none were treated with extracorporeal membrane oxygenation.
CONCLUSIONS
In summary, we describe the first utilization of high dose, intravenously infused, vitamin C in the treatment of severe gastric acid-induced ARDS. Both extensive preclinical work and as well as human studies thus far, examining parenterally administered vitamin C, leads us to conclude that this new form of therapy may become a useful adjunct in the care of patients with acute lung injury. The use of intravenous vitamin C is slowly emerging as a potentially effective adjunctive therapy for ARDS secondary to multiple etiologies (i.e., bacterial and viral infection). Bharara et al previously described the use of intravenous vitamin C as adjunctive therapy in a young woman with recurrent sepsis-induced ARDS,16 while Fowler et al reported intravenous vitamin C infusion in a patient who developed ARDS secondary to enterovirus/rhinovirus-induced ARDS.17 The current case report and the two reports cited here16,17 along with the phase II NIH-supported multi-center trial, examining intravenous vitamin C’s role as therapy in sepsis-induced ARDS will begin to shape the usage of vitamin C as a new standard of care therapy. As has been established for decades now, ARDS can occur secondary to many lung insults. In the current report, intravenous vitamin C was employed in a patient with who developed ARDS secondary to massive gastric acid aspiration.
DECLARATIONS
Ethics approval and consent to participate: This study was approved by the Virginia Commonwealth University Institutional Review Board (IRB). The IRB approval number assigned to this trial was: HM12903.
Consent for publication: Consent for publication was given by the patient. Data for this report was de-identified and confidentiality maintained.
ACKNOWLEDGEMENTS
The authors acknowledge the superior care provided by the VCU Cardiothoracic Nursing service for their amazing nursing care which assured a good outcome to this patient’s care.
COMPETING INTERESTS
The authors declare no financial or non-financial competing interests.
Availability of Data and Materials
Data for this case report was extracted from the patient’s electronic medical record and the VCU Radiology Imaging Service. The dataset generated and analyzed during the current study are not publicly available due to the fact that individual privacy could be compromised. The authors feel it essential that privacy not be compromised. We assure the material reported here be kept confidential to maintain that privacy. Thus, the data is NOT publically available due to confidentiality reasons.
AUTHORS’ CONTRIBUTIONS
– Christin Kim, MD: Clinical Critical Care of the patient, ECMO clinician, manuscript drafting, editing;
– Orlando Debesa, DO: Clinical Critical Care of the patient, ECMO clinician, manuscript drafting, editing;
– Patricia Nicolato, DO: Clinical Critical Care of the patient, Thoracic Surgeon who inserted ECMO, manuscript drafting, editing
– Bernard Fisher, MS: Vitamin C scientist, manuscript drafting, editing;
– Ramesh Natarajan, PhD: Vitamin C scientist, manuscript drafting, editing;
– Alpha A. Fowler, III, MD: Vitamin C scientist, manuscript Author.
FUNDING
National Institutes of Health, 5UM1HL116885-02, The Aubrey Sage Macfarlane Lung Injury Research Fund (a philanthropic fund gifted to the VCU Johnson Center for Critical Care and Pulmonary Research).
We additionally acknowledge the amazing care provided by the intensive care unit Nursing staff who cared for the patient in the Cardiac Surgery Intensive Care Unit at Virginia Commonwealth University.
TRIAL REGISTRATION
Trial registry: Clinical Trials.gov; https://clinicaltrials.gov/ct2/show/NCT02106975?term=citris-ali&rank=1, Trial Registration Number: NCT02106975, Date of Registration: March 27, 2014, Date of 1st enrollment: 9-15-14.










