INTRODUCTION
Diabetes mellitus (DM) has been recently a widespread disease. According to the last data of International Diabetes Federation (IDF), 463 million population has DM with increasing prevalence.1For adequate treatments, several standard guidelines of diabetic therapy have been found, in which American Diabetes Association (ADA) presented recent concept and therapeutic management in January 2021.2 DM has given public health burden from social and economic aspects. Furthermore, it also brings macroangiopathy and microangiopathy for diabetic patients. Among them, neurological complications include upper gastrointestinal (GI) symptoms and decreased motility because of impairment of adequate control of digestive system.3 In recent years, there have been various discussion concerning the complications of diabetes.4
From historical point of view, the usual diet therapy was formerly calorie restriction (CR), where fat food was mainly limited for reducing total calorie. After a variety of nutritional practice and research for decades, low carbohydrate diet (LCD) was introduced for medical and healthcare fields. It was Drs Atkins and Bernstein who had shown the effect for diabetes and developed the actual methods.5 In the light of evidence-based medicine, clinical effect of LCD was revealed by some large studies.6 The effective period for LCD has been more longer years7 and LCD has become recently rather prevalent in North American and European countries.
On the other hand, authors and co-researchers have initiated LCD in Japan and developed LCD treatment method so far.8 We have established Japan LCD Promotion Association (JLCDPA), and continued a variety of opportunities for original papers, books, medical societies, seminars and workshops.9 Further, various research related to LCD and CR have been reported, including meal tolerance test (MTT), insulinogenic index (IGI), continuous glucose monitoring (CGM), daily profile of blood glucose and Morbus (M) value.10,11 Recently, we have shown clinical application of Xultophy for type 2 diabetes (T2D) with some medical problems.12,13
Our diabetic team has continued to treat many diabetic patients for years.14 Among them, we have reported impressive diabetic cases with various complications. We experienced a case in which a T2D male patient showed remarkable efficacy for LCD. Furthermore, the case had some diabetic complications in the axis of digestive system, including diabetic gastroparesis (DG), occasional liver dysfunction suspected of probably related with DG. This case seems to be clinically impressive, and then general overview of the case and some discussion will be described in this article.
CASE REPORT
Medical History
The patient is a 64-year-old man, who had no remarkable illness when he was young. His work has been a Buddhist priest and always busy for long. He was diagnosed with T2D at the age of 42 and was subsequently treated with oral hypoglycemic agents (OHAs). He developed low back pain (LBP) seven years ago and was diagnosed with spinal stenosis. Then he received surgical treatment, followed by improvement. Recently, his diabetic control has been unstable, and then he was transferred to our diabetic department of the clinic.
Physical Examination
Consciousness is alert, and speech is normal and ordinary. Vitals showed pulse rate 60/min, and blood pressure 132/78 mmHg. His physique showed 177 cm in height, 74 kg in weight, 23.6 kg/m2 in body mass index (BMI) with ideal body weight (IBW) as 68.9 kg. Physicals showed that lung vesicular, heart no significant murmurs, abdomen soft and flat, no tenderness, bowel sound audible. Abdominal circumference is 87 cm, and thigh circumference was 43 cm at 10 cm position from upper border of patella. Neurological findings showed unremarkable.
Examination Results
The results of biochemical data at first visit were shown in Table 1. It showed normal liver function tests and abnormal data were not observed. Other examinations were as follows: Chest X-P was negative, and electrocardiogram (ECG) was within normal limits. The examination of pulse wave velocity (PWV) showed that ankle brachial index (ABI) 1.19/1.23, brachial-ankle PWV(baPWV)2453/2669. Echography of cervical artery revealed that maximum data of intima media thickness (IMT) 0.8/0.8 mm, plaque score (PS) 4.5 indicating slight arteriosclerosis (1.1<PS<5.0).
| Table 1. Biochemical Data |
| CBC |
GGT 48 U/L (<86) |
| Hb 14.1 g/dL |
ALP 196 U/L (80-260) |
| RBC 442 x 106 /μL |
LDH 179 U/L (124-222) |
| WBC 7100 /μL |
T-Bil 0.6 mg/dL (0-1.0) |
| Plt 26.9×104 /μL |
CK 124 U/L (41-153) |
| BUN 20 mg/dL |
Amylase 50 U/L (35-125) |
| Biochemical |
CRP 0.2 mg/dL (0-0.6) |
| Cr 0.9 mg/dL |
HBs Ag negative |
| Uric acid 6.8 mg/dL |
Diabetes-related |
| TG 144 mg/dL |
HbA1c 9.2% |
| HDL-C 50 mg/dL |
Glucose 278 mg/dL |
| LDL-C 83 mg/dL |
Urinalysis |
| TP 6.6 g/dL |
Protein (-) |
| Alb 3.9 g/dL |
Sugar (+) |
| AST 25 U/L (7-38) |
Urobilinogen (-) |
| ALT 23 U/L (4-44) |
pH 6 |
Clinical Progress
From mentioned above, current medical problems so far are summarized in the following: #1 T2DM, #2 hypertension, #3 arteriosclerosis of carotid artery. For basic treatment of diabetes, he was advised to start LCD. He was provided some medication for #1-3, which included sitagliptin phosphate hydrate (Januvia®) 50 mg 1 Tab, Voglibose (Basen®) 0.3 mg 3 Tab, diltiazem hydrochloride Retard-capsule (Herbesser-R®) 100 mg 1 Tab and aspirin (Biaspyrin®) 100 mg 1 Tab.
By continuing LCD for several months, his hemoglobin A1C (HbA1c) values and body weight were decreased. The clinical course is described in Figure 1. For 4-5 months duration of LCD, the HbA1c was normalized around 5.6-5.7%, and his clinical progress was stable. Actually, LCD has three levels, which are super-LCD, standard-LCD and petite-LCD. They contain the ratio of carbohydrate in calorie as 12%, 26% and 40%, respectively.15 He continued super-LCD for 3-months, standard-LCD for 3 months, and petite-LCD after that.
Figure 1. Clinical Progress Reduction of HbA1c and Weight and Occasional Liver Dysfunction are Found
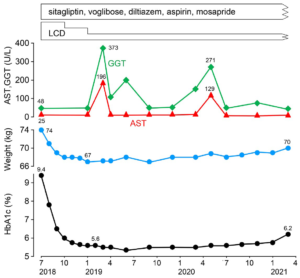
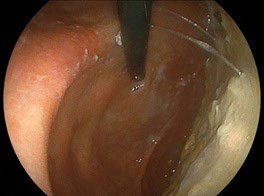
When the patient visited our clinic, we have checked echograph and abdominal computerized tomography (CT) scan for evaluating the abdominal organs in August, 2018. Abdominal CT scan showed the findings of multiple gall stone, dilation of the common bile duct and much amount of food residue in the stomach (Figures 2A and 2B). This finding means impaired gastric emptying and/or delayed gastric emptying (GE). The case is diabetic, then it is called as DG. Then, some questions concerning the complaints of digestive system were asked to the patient. The reply included some symptoms of functional dyspepsia (FD) persisting, such as heart burn, abdominal fullness and constipation. After that, upper gastrointestinal endoscopy was performed in September, 2018. The result showed the presence of extremely delayed gastric emptying (Figure 3). Before the exam, the case was ordered to keep fasting at least 13-hours from the previous night. He had spent usual life, and he did not have any episode of overeating or drinking much related to this. Consequently, the case has other medical problems summarized as follows: #4 gall stone, #5 FD, #6 DG. For the treatment of #4 and #5, mosapride citrate hydrate (Gasmotin®) 5 mg 3 Tab, and sennoside A and B calcium (Sennoside®) 12 mg, 2 Tab before sleep a day were started. The effect of the both was observed with relieve of symptoms.
Figure 2. Abdominal CT
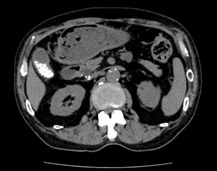
A. Transvers view: galls stone and
gastroparesis are present
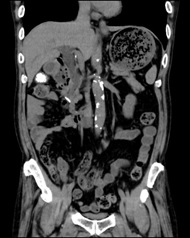
B. Coronal view: dilated common bile
duct are found
Figure 3. Endoscopic Findings: Much Food Residue is Found in the Stomach Due to Gastroparesis
During his clinical course, liver function was exacerbated twice, in March 2019 and in May 2020 (Figure 1). In the first time, it rose to AST 196 U/L, ALT 107 U/L, GGT 373 U/L, ALP 396 U/L, LDH 256. A similar episode was found in the second time, with similar changes in liver function tests, which seemed to be the medical problem of #7 occasional cholestasis-type liver dysfunction. In each episode, he did not have the episode of drinking much. He continued a usual life with no particular exacerbated gastrointestinal symptoms such as fatigue, abdominal pain, fullness, nausea, vomiting, constipation, abnormal bowel movements and jaundice. At that period, liver function-related tests in detail were conducted. The results showed that T-Bil 0.3 mg/dL, alpha-fetoprotein (AFP) <2.0 ng/mL (<20), Alb 58.2% (60.2-71.4), α1-Glb 2.2%, α2 -Glb 8.7%, β-Glb 9.3%, γ-Glb 21.6% (10.5-20.3), A/G ratio 1.3 (1.5-2.5) as proteinogram. These results also suggested the existence of # 8 chronic liver disease (CLD). No particular liver dysfunction has been observed since June 2020.
Another diabetic problem was found concerning retinopathy. He was pointed out to have retinopathy at the age of 56-years old. The diagnosis was proliferative retinopathy and he received laser treatment in the University Hospital. In February, 2019, he complained of blurred vision, then he was referred to the ophthalmology department of the university hospital. The important points of the diagnosis are summarized in the following. Both eyes have diabetic proliferative retinopathy. Previously, cataract surgery was performed on the both eyes and intraocular lens were inserted. Vitrectomy is performed on the right eye. Currently, the situation of retinopathy has been stable. The intraocular pressure was 18-20 mmHg for both eyes, and the visual acuity was 0.4 on the right and 1.5 on the left in the work of correction. Since the atrophy of macula is observed in the right eye, it is considered difficult to improve visual acuity function in the future. Consequently, he has suffered from # 9 proliferative retinopathy for long.
DISCUSSION AND CONCLUSION
This report presents an interesting case. The case is a 64-year-old man with T2DM who has a 22-year history of diabetes. He was referred to our clinic because of unstable control. HbA1c and body weight were reduced by LCD for a few months, associated with remarkable clinical effects. DG, gallstones and common bile duct dilation were found in abdominal CT scan. Upper gastrointestinal endoscopy examination showed much food residues in the stomach even after fasting for 13 hours. Furthermore, two episodes of occasional liver dysfunction were observed during the clinical course. Possible causes are involved in DG, gallstones, common bile duct dilation, constipation, overeating and overdrinking. As regards to the discussion, 1) LCD, 2) DG, and 3) liver dysfunction would be described in this order.
Firstly, LCD was applied to this case, which showed clinically significant efficacy. HbA1c decreased from 9.2 to 5.6%, and body weight decreased from 74 kg to 67 kg for 6-months. The results seem to be typical case for the clinical efficacy of LCDs. As to another data, TG values were TG 144 mg/dL, 62 mg/dL, 43 mg/ dL, 51 mg/dL, in 0, 3, 6, 9-months from LCD start, respectively. Remarkable reduction of TG is common clinical effects of LCD therapy.7 Authors and colleagues have continued LCD treatment for more than 2700 cases with diabetes, obesity and metabolic syndrome.16 The results showed that weight reduction was 6.6% in average, and 2.6-9.8% in the quartiles of 25-75% associated with mean weight reduction as 4.3 kg.
As regards to LCD, authors have managed to recommend three kinds of LCD in order to make everyone understand and practice easily. They are petite-LCD, standard-LCD and superLCD, associated with including carbohydrate ratio for 40%, 26%, and 12%, respectively.15 These three types have been actually useful to continue LCD therapy for medical care and healthcare. In current case, he has continued super-LCD at first for 3-months, successively standard-LCD for 3-months, accompanied by petiteLCD after that. This trial seems to be satisfactory associated with maintaining proper body weight.
Secondly, diabetic gastroparesis (DG) has been the important medical issue. Diabetic neuropathy has broader concept with signs and symptoms, in which various gastrointestinal (GI) symptoms are observed. This is so-called diabetic enteropathy (DE).17 Among DE, autonomic neuropathy show an crucial pathogenetic role, associated with reduction of interstitial cell and expression of neuronal NO synthetase.17 Those mechanisms bring to abnormal GI motility, leading some symptoms such as nausea, vomiting, dyspepsia and constipation. DE has been found in rather larger ratio of diabetic patients. They usually show upper GI symptoms and impaired motility.18 Regarding the motility, impaired GE is found about 50% of patients with T1D and T2D who are complaining of FD, diabetic gastroparesis (DG) and so on.19 Since dyspepsia and gastroparesis may show similar physio-pathological function and clinical signs, the differential diagnosis is not so easy. A recent report suggested that FD and DG may show different expressions, despite of the same spectrum of gastric neuromuscular dysfunction.20
Diabetic gastroparesis has been characterized for its upper GI symptoms, including nausea, vomiting, epigastric distress, bloating or early satiety, which show without organic obstruction. From cumulative proportions of gastroparesis for >10-years, hazard ratios (HRs) for gastroparesis to controls was 33 in T1D, and 7.5 in T2D. Further, gastroparesis risk in T1DM was higher than in T2D (HR 4.4), and T1D showed association of gastroparesis and heartburn more at baseline (HR 6.6).21 Although gastric emptying is often delayed in gastroparesis, overall changed motility does not correlate with the symptom severity.22
The term dyspepsia includes a set of symptoms with epigastric localization, which can be episodic or persistent, with variable intensity and severity. In the clinical setting, it is often difficult to characterize these symptoms and to distinguish dyspepsia from other GI disorders such as gastroesophageal reflux disease (GERD).23 The American College of Gastroenterology (ACG) and Canadian Association of Gastroenterology (CAG) clinical guidelines give a useful definition of dyspepsia as predominant epigastric pain which lasts at least one month and is associated with any other upper GI symptom such as epigastric fullness, nausea, vomiting or heartburn.24
Regarding the treatment of diabetic enteropathy and/or DG, three options are present.18 They are i) proper dietary modification, ii) restriction of glucose intake with better glucose control, iii) the first-line of pharmacological therapy such as the use of prokinetics. For treatment of GE, there are serotonin type 4 (5-HT4) receptor agonists such as mosapride, cisapride that is also effective for reduction of abdominal pain.25 Furthermore, dopamine-2 receptor antagonists are also present such as metoclopramide that is effective for reduction of early satiety, nausea and vomiting. As a 5-HT4 receptor agonist, mosapride citrate hydrate (mosapride r) has been known to show beneficial effect and to enhance GE.26 Mosapride combined with a Proton Pump Inhibitor (PPI) significantly improved the reflux symptom score compared with that of PPI alone.27
Thirdly, occasional liver dysfunction twice during the clinical course were observed (Figure 1). What kind factors may be involved in these episodes? Some possible triggers would be gall stone, enlarged volume of stomach due to DG, overeating, overdrinking, and other factors. We had taken detail history of daily life from the patient, but he did not notice particular changes in his status. The gall stone was silent for symptoms, showing no abdominal pain or discomfort in right upper quadrant. CT findings showed some bile duct enlargement, which may be related the presence of gall stone. Gallstones seemed to be small cholesterol stones, and the possibility of bile duct cancer will be followed and checked hereafter. There was a possibility of the relationship of gall stone and occasional liver dysfunction. However, responsible causes have not be clarified yet. Consequently, this case will be followed-up carefully from several points of view.
There are some limitations in this report. The case is limited one case with DG and occasional liver dysfunction. In detail, he seemed to show always delayed gastroparesis and two episodes of liver dysfunction. Its pathophysiological mechanism and situation would be considered associated with several factors, such as medical history, diabetic complication, and interactions in digestive system.
In summary, a patient with T2D was described with several medical problems. He showed satisfactory reduction of HbA1c and weight by LCD, GI tract problems such as diabetic gastroparesis, and occasional liver dysfunction. This impressive report will hopefully become a reference for developing diabetic practice and research.
ETHICAL CONSIDERATIONS
This research study was conducted in compliance with the proper ethical principles, which is based on the Declaration of Helsinki. In addition, some commentary was present for the Ethical Guidelines for Research in the medical field for Human beings and in the conduction of the good clinical practice (GCP). Authors have taken the written informed consent from the patient in this research. Furthermore, authors established the ethical committee for the clinical research in Kanaiso Hospital. The members were the president, the vice-president, and the director of Pharmacology department, the headnurse, and experts in the medical and legal specialties. We have fully discussed the detail of the research content and made confirmation that this research will be adequate and agreed with all members.
FUNDING
There was no funding received for this paper.
CONSENT
The authors have received written informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









