INTRODUCTION
Type 1 diabetes (T1D) mellitus is an autoimmune pro- cess that selectively destroys the insulin-producing beta cells in the islets of Langerhans, resulting in an absolute deficiency of insulin.1 The International Diabetes Federation (IDF) recently reported that the worldwide incidence of T1D affects nearly 500,000 children under the age of 15 years.2 The largest reported numbers can be found in Western Europe and North America.3 However, the highest mortality rate due to T1D in children is highest in sub-Saharan African countries such as Sudan, which shows 42.6 deaths per 100,000 children less than 15 years of age while in the United States, the incidence is 0.63 per 100,000 children.4 It is clear that T1D is a serious health priority through- out the world.
Most recently, progress has been made in determining cellular malfunctions in patients with T1D so that effective treatments can be developed. Scientists from the Clinical Sciences Centre (CSC) in West London have shown that microRNA 375 is released into the bloodstream in large quantities as soon as the pancreas cells that produce insulin begin to die.5 In addition, scientists from Joslin Diabetes Center at Harvard Medical School have discovered higher levels of miR-200 protein and consequently impaired Deoxyribonucleic acid (DNA) repair in stem cells from T1D patients with severe complications compared to T1D with absent or mild complications.6 Their next step will be to determine whether these molecules can be detected in the bloodstream and used reliably as effective bio-markers for T1D diagnosis and complications.
The rising prevalence of T1D and its vascular complications are a major cause of concern, especially regarding the development of serious diseases that affect the kidneys, nerves, cardiovascular system, and the eyes. Diabetic retinopathy, one of the most common complications of T1D, is classified as micro angiopathy affecting the retinal vasculature, including microvascular leakage from collapsing inner blood-retinal barrier (retinal oedema) and microvascular occlusion.7 Reports suggest that individuals with T1D should undergo a retinal exam after 5 years of onset or by 15 years of age to screen for and prevent retinopathy.8 Retinal hemorrhages are often associated with long history of diabetes9 and high blood glucose as well as long duration of elevated blood pressures.10 Other than glycemic and systemic BP control, no other biochemical factors have conclusively been demonstrated to protect against diabetic complications.11
The target fasting BG and HbA1c are 80-130 mg/dL and <7%, respectively.8 The target BP is <130/80.12 Loss of control of these important health parameters in a child with T1D results in progressive retinopathy.13 Research has established the importance of BG control to prevent development and progression of diabetic retinopathy. Prolonged exposure to hyperglycemia induces a large number of cellular level alterations in vascular tissue, including non-enzymatic glycosylation of proteins and lipids and increased oxidative stress14 in addition to substantially increased retinal oxygen consumption.15 BP control is being advocated for the same purpose, as patients with T1D and uncontrolled hypertension are twice as likely to have reduced vision when compared to patients with diabetes and controlled BP.16
MATERIALS AND METHODS
Protocol for Screening
Visual Acuity (VA) and Intraocular pressures (IOP) were performed using an automated VA instrument from Canon RK-F2 Full Auto Ref-Keratometer (Tokyo, Japan) and Canon TX-20 Full Auto Tonometer (Tokyo, Japan) to capture pertinent data. BP was measured manually using Korotkoff technique while the patient sat comfortably.
The use of a Canon CR2 Plus AF (Tokyo, Japan) non- mydriatic retinal imaging system with a 18 Mp Complementary Metal Oxide Semiconductor (CMOS) sensor provided color and Fundus autofluorescence (FAF) fundus images. Image management and post processing of digital imagery was done using Canon’s proprietary software, image SPECTRUMTM V 3.01 (Ir- vine, California, USA), to manage all electronic data including VA, tonometry as well as importing OCT and OCTA images for use in tele-reading.17
A Spectral Domain (SD)-OCT provided high-resolution structural imaging of sequelae of posterior segment manifestations in the subject’s previously healthy eye. The following year, the subject was re-imaged using an optical coherence to mography angiography (OCTA) (Optovue, Fremont, California, USA) in addition to the previous imaging equipment. A certified diabetic reader quantified the extent of retinopathy and its progression over time by performing retinal hemorrhage count. TeamViewerTM (Tampa, Florida, USA) was used to perform re- mote tele-presence tele-ophthalmology.
Case Presentation Results
A 20-year-old Hispanic male with 15-year history of T1D has been followed remotely by our University based Tele- medicine (Rutgers New Jersey Medical School in Newark, NJ, USA) during the annual meetings of Friends for Life (FFL): International Children with Diabetes (Orlando, Florida, USA: http://www.childrenwithdiabetes.com) for the past 5 years, be- ginning in 2010. FFL provides education and support for children with diabetes and their family members in order to maintain a healthy, typical lifestyle with diabetes.
The subject was diagnosed with T1D DM in 1999 after experiencing flu-like symptoms, and treated with a Medtronic insulin pump (Minneapolis, Minnesota, USA). In 2010, he attended friends for life (FFL) and participated in a comprehensive ophthalmic screening. During his initial visit, we noted a BG of 184 mg/dL and BP of 128/78. (Figures 1 and 2) A family history revealed the presence of type 2 diabetes (Father) and hypertension (Mother and Father). His retinal images were free of retinopathy with no hemorrhages and showed no signs of hyperglycemic or hypertension damage. (Table 1, Figure 3)
Figure 1: The subject’s fasting blood glucose levels are recorded in real time evaluation.
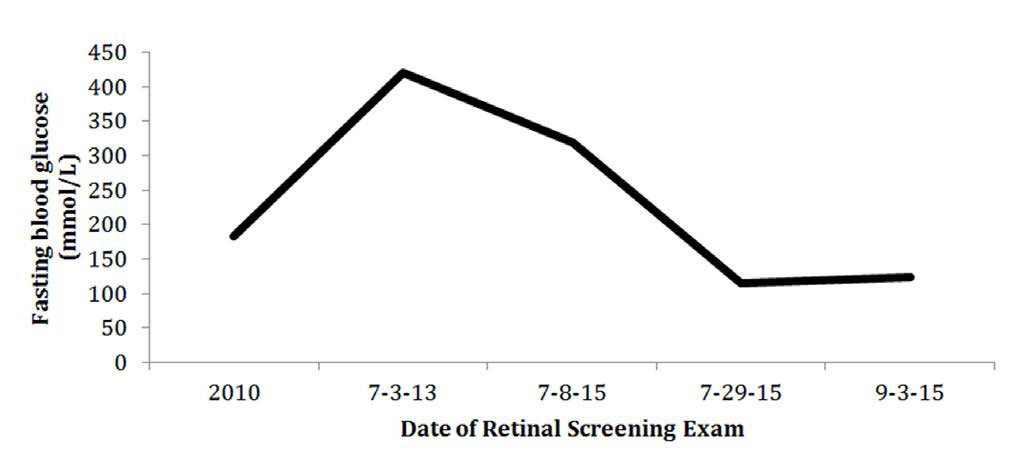
Figure 2: The subject’s blood pressure values are recorded in real time evaluation.
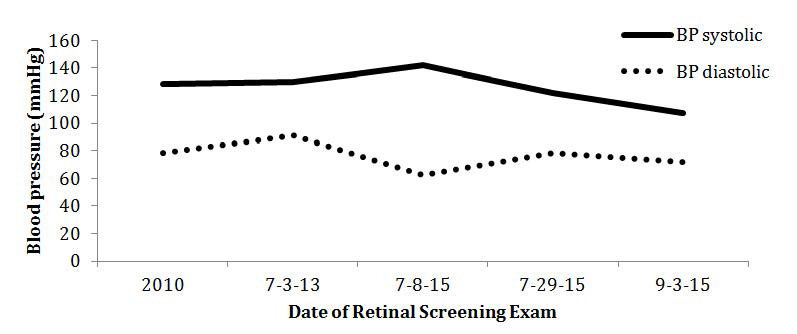
Figure 3: The subject’s total flame and dot hemorrhages in the left eye are recorded in real time evaluation.
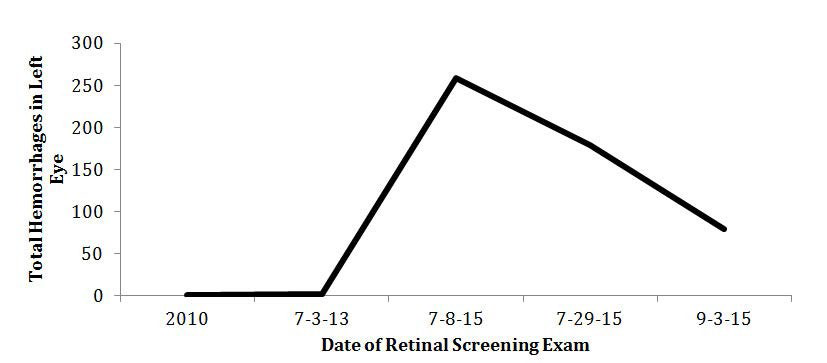
Figure 4: The subject’s CFP was imaged in 2013. A 45-degree view of the subject’s left eye posterior pole identified a few dot-blot hemorrhages (example as shown by arrow) and a single IRMA. No diabetic retinopathy was detected.
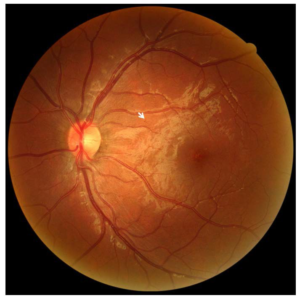
Figure 5: The subject’s HbA1c levels are recorded in real time evaluation.
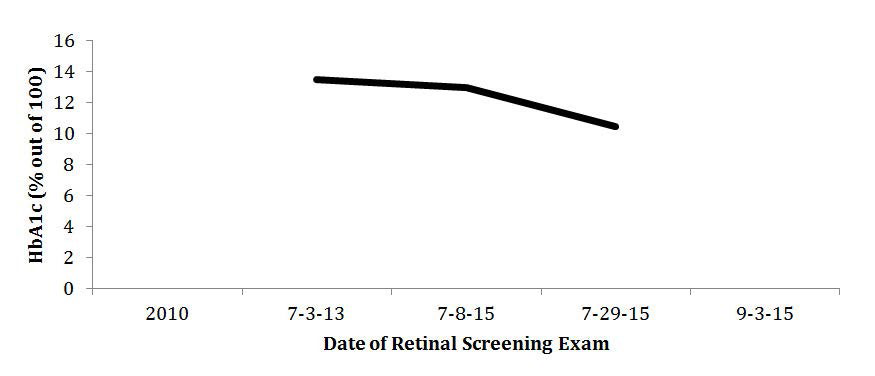
Table 1: The subject’s left eye retinal fundus images were evaluated for counted hemorrhages and IRMAs. His BP, fasting BG, last HbA1c, VA of his left eye, and duration of T1 DM history were also noted.
|
Left eye
|
Jul 2010 |
Jul 11, 2013 |
Jul 8, 2015 |
Jul 29, 2015 |
Sept 3, 2015 |
| Dot hemes |
0 |
2 |
221 |
168 |
77
|
|
Flame hemes
|
0 |
0 |
38 |
11 |
2 |
| IRMAs |
0 |
1 |
12 |
10 |
13
|
|
T1 DM history (years)
|
10 |
13 |
15 |
15 |
15 |
| BP (mmHg)v |
128/78 |
130/91 |
142/62 |
122/78 |
107/72
|
|
HbA1c (%)
|
n/a |
13+ |
13.5 |
10.5 |
n/a |
| BG (mmol/L) |
184 |
421 |
319 |
115 |
124
|
|
VA (left eye) VA (left eye)
|
20/80 uncorrected |
20/30 corrected |
20/30 corrected |
20/30 corrected |
n/a
|
The subject returned three years later in 2013 to the annual FFL meeting when he was 18-years-old and underwent a follow-up comprehensive ophthalmic screening. At the time he was taking fast-acting Novolog with pump but would occasion- ally use long-acting Lantus when not on the pump to control his T1D. His Body Mass Index (BMI) was 19.8, and he was within normal weight range. His self-reported BG was 421 mg/dL and last HbA1c was 13%. (Table 1, Figures 1,3 and 5) His blood pressure was 130/91 with a pulse of 106 bpm. (Table 1, Figure 2) Fundus images of the left eye showed 2 small dot hemorrhages, no flame hemorrhages, and a single IRMA, (Figures 3 and 4, Tables 1 and 2) as interpreted by a certified diabetic reader.
In January 2015, his pediatrician placed him on Enalapril (Kenilworth, NJ, USA) BP medication control. However, after only taking the medication once, the subject decided not to continue the treatment as he felt the medicine resulted in numb- ness and pain in his legs. During that time, he also halted use of his automated Medtronic insulin pump as he felt he was experiencing discomfort and dermal allergic reaction to the plastic tubing. He reverted to self sub-cutaneous injections 3 times per day. During that period he reported an uncontrolled diet consisting of mostly of fast food due to his busy life style.
On February 27, 2015, the subject underwent a full diabetic eye examination in a private optometric clinic in Paramus, NJ using a biomicroscope slit lamp and both 90-diopter and 20-diopter lens. No retinopathy was noted. The medical plan was for the subject to revisit in 6 months for retinal reevaluation and to see his ophthalmologist should he notice any sudden vision changes.
One hundred and thirty-one days following his most recent diabetic eye examination with now a history of 15 years T1D DM, the subject participated in FFL ocular screening. We noted his BP was 142/62 mm Hg and his fasting BG was 319 mg/dL while his May 2015 HbA1c was 13.5% (Table 1, Figures 1, 2 and 5). We noted a sudden change in his retinal findings of the posterior pole (45 degrees) consistent with diabetic retinopathy. A total of 221 dot hemorrhages, 38 new flame hemorrhages, and 12 IRMAs were counted in the left eye by a certified diabetic reader. (Figures 3 and 6, Tables 1 and 2) A wide field retinal image of his undilated eye was ordered for his left eye with four Early Treatment Diabetic Retinopathy Study (ETDRS) fields of 45 degrees each. An automated montage was created using image SPECTRUMTM seen in Figure 7. This allowed for more comprehensive remote tele-consultation, evaluating the extent of hemorrhages as well as stress on the retina caused by the un- controlled elevated BP. Retinal montage shows the majority of retinal hemorrhages and vascular changes appeared in the posterior pole and were mostly absent in the periphery of the wide field image. Flame hemorrhages are associated with high BP and could be seen mostly within the arcades along with retinal stress.
Tele-consultation was conducted in accordance with Digital Imaging and Communications in Medicine (DICOM) and Health Insurance Portability and Accountability Act (HIP- PA) protocols from Orlando, Florida, USA with our primary lo- cation at University Hospital with board certified ophthalmologists in Rutgers, New Jersey, USA. Team ViewerTM software was configured to share and review the subject’s retinal images including color, red free, FAF, OCT, and OCTA. We used a cell phone connection to discuss the details of VA, IOP, BP, glucose levels as well as the subject’s history. His identifiers were never used during communication or through use of TeamViewerTM. The retinal findings were classified as severe NPDR as defined by the presence of at least one of the following: venous beading, IRMAs, or hemorrhages/microaneurysms present in a standard 45 degree field of view of the posterior pole photograph in at least two quadrants. (Figure 6, Table 2)18
Figure 6: The subject’s CFP was imaged on July 8, 2015 (131 days after his last diabetic eye examination, which had no reported retinopathy). A 45-degree view of the left eye posterior pole identified a significant number of dot-blot hemorrhages, flame hemorrhages (example depicted by arrow), and IRMAs. A board certified ophthalmologist diagnosed the patient with NPDR
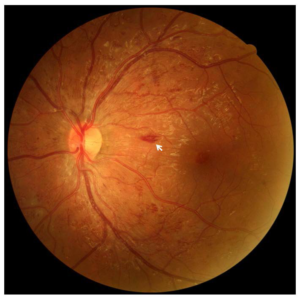
Figure 7: The subject’s left eye CFP automated montage was created on July 8, 2015. ETDRS fields of 45-degree view from each quadrant were used to create a wide field retinal image. The hemorrhages and vascular changes were centrally located and noticeably absent in the periphery of the wide field image.
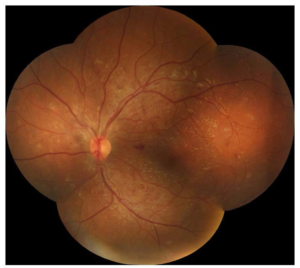
Table 2: The subject’s left eye retinal fundus images were evaluated prior to NPDR diagnosis, on the day of medical NPDR diagnosis, 21 days following NPDR diagnosis, and 57 days following NPDR diagnosis. A board certified diabetic reader counted the number of flame and dot hemorrhages as well as IRMAs in the various regions.
|
Location
|
Dot hemorrhage |
Flame hemorrhage |
IRMA |
Total micro-vascular changes |
|
July 11, 2013
|
|
45° posterior pole
|
1 |
0 |
0 |
3 |
| Outer inferior |
0 |
0 |
0
|
|
Outer superior
|
1 |
0 |
0 |
| Total |
2 |
0 |
1
|
|
July 8, 2015-131 days following last diabetic retinal exam; initial NPDR diagnosis
|
| 45° posterior pole |
108 |
25 |
3 |
271
|
|
Outer inferior
|
40 |
4 |
3 |
| Outer superior |
73 |
9 |
6
|
|
Total
|
221 |
38 |
12
|
|
July 29, 2015- 21 days post NPDR diagnosis
|
| 45° posterior pole |
98 |
8 |
3 |
189
|
|
Outer inferior
|
34 |
1 |
3 |
| Outer superior |
36 |
2 |
4
|
|
Total
|
168 |
11 |
10
|
|
September 3, 2015- 57 days post NPDR diagnosis
|
| 45° posterior pole |
48 |
2 |
3 |
92
|
|
Outer inferior
|
6 |
0 |
4 |
| Outer superior |
23 |
0 |
6
|
|
Total
|
77 |
2 |
13
|
A board certified retinal specialist remotely interpreted the OCT and OCTA images. He reported that the OCTA showed areas of retinal telangiectasia and occasional micro aneurysm formation. (Figure 8) Review of the digital CFP revealed hemorrhages associated with diabetic and hypertensive retinopathy. (Figure 6) However, the microvascular changes seen in the OCTA (Figure 8) were not recognized in the CFP (Figure 6) and offered further insight into the extent of retinopathy than the CFP suggested.
Figure 8: The subject’s left eye (OS) OCT-A image was taken 131 days after his last diabetic eye examination, which had reported no signs of retinopathy. The left image depicts microvascular changes (examples highlighted by arrows) consisting of retinal telangiectasia and occasional micro aneurysm formation. This shows that the hemorrhages and microvascular changes noted in the CFP could be due to hypertension and not diabetic retinopathy. The right image shows that there is no macular edema but there were some vascular changes. These details were not seen in CFP.
After confirming the potential urgency for vision loss and end organ damage, the subject was scheduled to be seen by his primary physician and ophthalmologist in NJ 21 days after the Orlando retinal screening because the subject resides in New Jersey. He was directed to resume taking daily Enalapril BP medicine immediately and to strive to greatly reduce his Hb1AC (from >13%).
On his first visit to University Hospital (UH) in Newark (21 days after FFL 2015), the subject maintained his VA at 20/30 in both eyes and had reduced his BP from 142/62 to 122/78 after resuming Enalapril medication. According to his neurologist, the tingling leg sensation that had caused the subject to cease medication was attributed to peripheral neuropathy onset. His July 2015 Hb1AC was reported to be 10%, and his BG was 115 mg/dL after he had made significant changes to his daily diet. (Table 1, Figures 1 and 5) His left eye seen in Figure 9 showed that the central flame hemorrhage had resolved and his retinal hemorrhages had reduced from 259 dot hemorrhages to 179 dot hemorrhages. (Figure 3, Tables 1 and 2)
Figure 9: The subject’s CFP was imaged 21 days following NPDR detection. A 45-degree view of the posterior pole in the subject’s left eye showed that the central flame hemorrhage had resolved and there were 30% fewer total retinal hemorrhages since NPDR diagnosis. An example of an IRMA is demonstrated by the arrow.
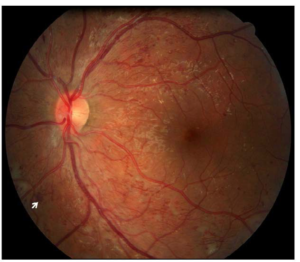
On his second visit to UH (57 days after FFL 2015), the subject had a BP of 107/72 while his fasting BG was reported to be 124 mg/dL (Table 1, Figures 1 and 2). His left eye was imaged (Figure 10) and showed his retinal hemorrhages had reduced to 79 hemorrhages. (Figure 3, Tables 1 and 2) His newly imaged OCT showed absence of macular edema and minimum foveal swelling. (Figure 11)
Figure 10: The subject’s CFP was imaged 57 days following NPDR detection. A 45-degree view of posterior pole in the subject’s left eye showed approximately 70% fewer counted retinal hemorrhages since diagnosis of NPDR
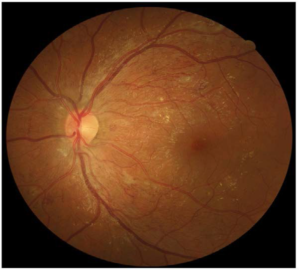
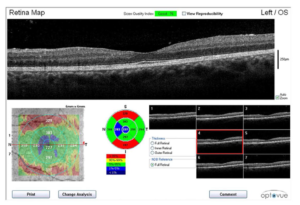
Figure 11: The subject’s left eye OCT was imaged 57 days following NPDR detection. The OCT wellness report demonstrated in monochromatic scans that there is an absence of macular edema. The bottom-left 6 mm x 6mm scan illustrates thickening of the macular tissues represented in red. The top and bottom right images show that the fovea has minimum swelling. Close observation needs to be carried out on a 30-day basis.
DISCUSSION
Types of Retinal Hemorrhages and Microvascular Changes
There are two main types of posterior pole hemorrhages and a microvascular change that can affect patients with long duration or poorly controlled BG and hypertension.
Dot hemorrhages (Figure 4) consist of small, bright red dots seen within the inner nuclear, the outer plexiform, and the outer nuclear layers. After a 15-year medical history of T1D, many develop these dot hemorrhages when the walls of thin retinal vessels are weakened and rupture.19 Due to their deeper location, dot hemorrhages take a longer time to resolve and affect pre-venular capillaries, thus signaling the presence of venous congestive disease.20
Flame hemorrhages (Figure 6) occur in the inner layer of the nerve fiber layer, and are mostly associated with severe hypertension that remains chronically elevated.21,22 These hemorrhages exhibit a characteristic flame shape resulting from blood traveling through axons of the ganglion cells.23 They are most frequently seen within the posterior pole, the area between the optic disc and the macula, and affect the superficial and peri- papillary capillary beds within the retina.24 Due to their superficial nature, they tend of resolve within six weeks when blood pressure returns to normal.25
In the more advanced retinopathy, intraretinal micro- vascular anomalies (IRMAs) (Figure 9) can be observed as dilated, tortuous capillary loops.26 They are found in areas of non-perfusion and typically connect retinal arterioles with retinal venules.27 These changes are indicative of severe non-proliferative diabetic retinopathy that may progress to proliferative diabetic retinopathy.28
With the progression of microvascular diabetic complications, tight BG control is important in the management of retinopathy. It is recommended that close to normal glucose levels should be maintained in patients in the earlier stages of diabetes.28 However, overall management of T1D by controlling high blood pressure may be as important as high BG levels,29,30,31,32 as hypertension causes more severe retinal hemorrhages that can be remediated with antihypertensive therapy after a few months.33,34
Hypertension and Diabetes
Hypertension (HTN) is a common comorbidity in diabetes, and is present in 20-60% of the diabetic population, which is 1.5-3 times higher than the age-matched non-diabetic population.35 HTN damage is associated with potentially devastating outcomes, including both macrovascular and microvascular complications.36 Several studies have sought to encode the ideal blood pressure goal for the diabetic population.
The UK Prospective Diabetes Study (UKPDS) demonstrated the significant reduction in microvascular events, primary retinopathy, seen in patients with high blood pressure control (goal<150/85 mm Hg) as compared to a more lenient regimen (goal<180/105 mm Hg).37 The Hypertension Optimal Treatment (HOT) study suggested that patients with diabetes benefit from a blood pressure goal of diastolic≤80 mm Hg compared to diastolic BP≤90 mm Hg; individuals who maintained the diastolic BP≤80 mm Hg experienced a 51% reduction in major cardiovascular events.38 The African Centre for the Constructive Resolution of Disputes (ACCORD) trial declared the lack of improvement in clinical outcomes with intensive blood pressure treatment (systolic<120 mm Hg) when compared to standard treatment goals (systolic<140 mm Hg).39 As a result of these studies, the cur- rent established blood pressure goal for patients with diabetes is systolic <130 mm Hg and diastolic <80 mm Hg.8 This is the goal used for the management of all diabetics, as there is limited evidence supporting the value of different blood pressure goals amongst T1D and T2 diabetics.
Management of BG and BP in patients with diabetes can quickly get out of control following puberty. For T1D adolescents and young adults, there is frequent failure to achieve good glycemic control, and it has been reported that mean HbA1c is highest during the ages of 11-19.40 In addition, hyper- tension is established as an independent risk factor for diabetic retinopathy, rather than by association with nephropathy.41 It has been reported that patients with diabetes who have uncontrolled hypertension double their risk for reduced vision as compared to patients with diabetes and controlled BP.42
The duration of diabetes is one of the strongest predictors for progression of retinopathy. It has been reported that the prevalence of retinopathy is 80% in those who have had a 15- year history of T1D.43 If the macula is not involved, retinopathy can go unnoticed during this critical 15-year time period. How- ever, the co-existence of uncontrolled hypertensive and long- term diabetic retinopathy further increases the possibility for rapid vision loss.44 Once the macula is involved, central vision may deteriorate rapidly and visual prognosis must be evaluated on an individual basis.
RESULTS
Of the 320 patients that were screened during FFL 2015, 43 patients (13%) had a history of T1D for equal to or greater than 15 years. Of the 43, eight patients (19%) also had hypertension (BP>130/80). Out of 8, 7 patients (88%) had HbA1c<12%.
Our subject was exceptional in that although he had been seriously counseled for at least 2 years since FFL 2013 regarding his hyperglycemia (HbA1c=13.5%) and hypertension (BP=142/62), he failed to follow a more judicious lifestyle as proposed by the FFL educational program. In 2013, with a 13-year history of T1D, he did not have retinopathy. Two years later in 2015, with a 15-year history of T1D and continued un- controlled BG and BP for >2 years, it took 131 days for severe NPDR to result. After regaining BP and BG control for >8 weeks (57 days) following NPDR diagnosis, the patient demonstrated signs of microvascular remediation through fewer counted dot and flame hemorrhages.
CONCLUSION
The subject had maintained good control of type 1 diabetes through most of his life, including the difficult phases of puberty. His decisions to cease tight control of BG and not follow the direction of his pediatric endocrinologist to take medication to control his high BP contributed to severe NPDR, as well as the peripheral neuropathy that he currently experiences in his feet.
T1D is a lifelong concern that can affect the psychological well being of affected children, as well as all members of a family providing lifelong self-care. However, our data suggests that education, tight control of all aspects affecting diabetes, support from family members, as well as groups such as FFL help maintain a healthier and normal lifestyle with diabetes.
Although we have seen promising return to better control of the subject’s diabetes and clearing of nearly 70% of his retinal hemorrhages from his severe NPDR diagnosis, some damage may be end organ reversible over time, while other dam- age may become permanent. Further studies in T1D, specifically looking at the role of controlled hypertension combined with hyperglycemia in patients who have retinopathy or long history of T1D are needed. Medical management of patients with T1D should include blood pressure monitoring at each ophthalmic clinical visit in order to help detect inappropriately fluctuating and high blood pressure patterns. Early detection of microvascular and macrovascular changes using digital retinal imaging along with OCT and OCTA can help detect various types of hemorrhages and vascular changes before symptoms of permanent vision loss manifest.
CONFLICTS OF INTEREST
No authors have conflicts of interest.
CONSENT
Consent was obtained at the time of examination.
















