INTRODUCTION
Rheumatoid Arthritis (RA) is a chronic inflammatory and immune-mediated disease with both articular and extra-articular manifestations. It is a disease of substantial morbidity and increased mortality, but within the last 2 decades has witnessed significant breakthroughs in our understanding of the disease. Advances in our understanding of RA have facilitated the development of pharmacologic therapies which make it possible to arrest disease activity for many patients. Attaining this achievable goal rests on the ability to accurately measure RA disease activity. In both scientific trials and clinical practice a variety of disease activity indices have been used to measure RA disease activity. These indices utilize information obtained from the patient, the physician, and/or serologic markers of inflammation. Despite access to and endorsement of such disease activity indices, a large portion of US rheumatologists do not routinely use indices in clinical practice.1 Multiple factors likely contribute to this trend, with the time demands of clinical practice an important consideration. In a robust clinical practice disease activity indices that require complex formulas to calculate a score or several minutes to complete hinder efficiency. Under these circumstances, an instrument such as a patient administered questionnaire that is easy to complete, easy for the provider to score, and minimizes or avoids serologic data gathering would be optimal. Additionally, a questionnaire focusing on upper extremity function would also be desirable. Good upper extremity function is critical to independent daily living.2 A questionnaire assessing upper extremity function would also correlate with the data gathered on physical examination of patients with RA as 26 of the 28 joints counted assess upper extremity disease activity.
The purpose of this study was to assess the reliability and internal consistency of the QuickDASH in the assessment of RA disease activity as compared to other disease activity indices. The Routine Assessment of Patient Index Data 3 (RAPID3) was the chosen “benchmark” questionnaire against which to compare the performance of the QuickDASH. The RAPID3 is a self administered questionnaire of disease symptoms for patients with RA.3 Scoring is composed of American College of Rheumatology (ACR) core set measures used to assess effectiveness of disease modifying therapy.3 The RAPID3 has also been endorsed by the ACR Working Group for the measurement of RA disease activity because it is sensitive to change, discriminates well between disease activity states, has remission criteria, and is feasible to perform in routine clinical care.3 Also of interest was how well the QuickDASH correlated with other RA disease activity indices or measure, specifically the Disease Activity Score 28 (DAS28), subject visual analogue pain assessment (VAS), swollen joint count (SJC), and tender joint count (TJC).
METHODS
Patient Population
This prospective cohort study commenced in July 2014 when patients receiving routine clinical evaluation of their RA were asked to complete the QuickDASH and RAPID3 at each clinic visit. All subjects received routine care for their RA at a single academic community hospital. Data analysis was performed in January 2016. The RAPID3 questionnaire was the standardized disease activity measure utilized by the clinic prior to the start of the study. In addition to data collected by the QuickDASH and RAPID3 questionnaires at each visit, the SJC, TJC, and subject visual analogue pain assessment (VAS) on a scale from 0 to 10 (0 implying no pain, and 10 implying the worst pain possible) were also collected at nearly every visit. At the majority of visits an Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP) were gathered and used to calculate a Disease Activity Score (DAS). The 28 joint DAS was calculated based on established equations incorporating the TJC, SJC, patient’s global assessment of pain, and ESR or CRP. ESR and CRP were measured by our hospital lab, in accord with established techniques for these two tests.
Subjects in this study were all 18 years of age or older who had a diagnosis of Rheumatoid Arthritis made based on the fulfillment of either the 1987 or 2010 ACR Classification Criteria for the disorder, depending on the year in which they were diagnosed. Seronegative RA subjects had either extra-articular manifestations typical for RA, radiographic or magnetic resonance imaging (MRI) of the hands which demonstrated synovial inflammation or erosions consistent with a polyarticular symmetric small joint arthritis favoring RA, or were diagnosed by another rheumatologist with RA but received routine care at this treatment facility.
Disease Activity Indices
The RAPID3 is a self administered questionnaire of symptom severity related to RA disease activity. A subset of 10 core variables found in the Multidimensional Health Assessment Questionnaire (MD-HAQ) are graded on a Likert scale ranging from 0 (without any difficulty) to 3 (unable to do). The patient also provides a global assessment of pain (score ranges from 0, no pain to 10, pain as bad as it could be) and a global assessment of health (score ranges from 0, very well, to 10, very poorly). The Likert scores for the 10 items from the MD-HAQ are added together, divided by 3.33, and then added together with the patient global assessment for pain and health. A composite “raw” score ranges from 0 to 30, a weighed score divides the “raw” score by 3 (for a scale of 0 to 10). Disease activity recall is over the week prior. Established disease cutoffs for the “raw” score are 0-3.0 for remission, 3.1-6.0 for low disease activity, 6.1-12.0 for moderate disease activity, and 12.1-30 for high disease.3 In this study, only RAPID3 “raw” scores were reported.
The Disabilities of the Arm, Shoulder, and Hand (DASH) is a 30 item, self administered questionnaire with 6 items related to symptoms and 24 related to function.2 Items are scored on a Likert scale, with 1 meaning no difficulty or symptoms and 5 meaning extreme symptoms or inability to do.2 Total scores range from 0 (no symptoms/full function) to 100 (maximal symptoms/no function). The time to complete the DASH is 4 minutes.2 A shortened version, the QuickDASH, was introduced several years after the DASH. It is an 11 item, self administered questionnaire with 3 items related to symptoms and 8 items related to function. Items are again graded on a 5 point Likert scale and the total score again ranges from 0 (no symptoms/full function) to 100 (maximal symptoms/no function). Disease activity recall for both the DASH and QuickDASH are the week prior. Neither the DASH nor the QuickDASH require obtaining serologic data or joint counts. Both the DASH and the QuickDASH are region specific, useful in assessing polyarticular conditions or measuring symptoms and function of the entire upper extremity.2
Statistical Analysis
To validate the QuickDASH in the assessment of RA disease activity, measures such as reliability and internal consistency were assessed. Reliability is the ability of a test to yield the same result on repeated trials under similar conditions. The test-retest reliability was assessed by the interclass correlation coefficient (ICC) and graphically in a Bland-Altman plot. Internal consistency was assessed by Cronbach’s alpha. An alpha of 0.7 conveys a fair degree of internal consistency, 0.8 conveys good internal consistency, and 0.9 excellent internal consistency.4 The concurrent validity of the QuickDASH to RAPID3 was assessed by correlation and Bland-Altman plot with adjusted unit of RAPID3.
This study approved by the hospital Institutional Review Board before data collection began with informed consent waived. All RAPID3 and QuickDASH questionnaire values were manually entered in an Excel spreadsheet with formulas used to calculate the scores. Accuracy of the RAPID3 scores were routinely verified with the worksheet available through the American College of Rheumatology, Atlanta, Georgia, USA.5 Statistical analysis was performed using SPSS (version 22; SPSS Inc, Chicago, IL, USA). A p value<0.05 was considered statistically significant.
RESULTS
Subject demographics are detailed in Table 1. Longitudinally over an 18 month period, an average of 6 time-points from 105 subjects were collected. The average age of study participants was 62.8±14.2 years old. About 70% of study participants were female and 74.1% were Caucasian. Just over 90% of subjects were actively treated with either a disease modifying medication and/or biologic response modifier during the course of this study. RA disease morbidities are shown in Table 2. Over 70% of the subjects were seropositive (had a serologic test result above the manufacturer reported upper limit for either Rheumatoid Factor and/or anti-cyclic citrullinated peptide IgG). Almost 75% had erosive changes on radiographic imaging or MRI, 37% had deforming disease, and 18% had extra-articular manifestations of either nodulosis or interstitial lung disease. Sixteen percent carried a diagnosis of RA for less than 2 years.
Table 1: Subject demographics.
Key: SD = Standard Deviation.
|
Age (mean±SD)
|
62.8±14.2 years old |
| Female : Male |
74 : 31
|
|
Ethnicity
|
|
| Caucasian |
78 (74.3%)
|
|
African American
|
18 (17.1%) |
| Hispanic |
3 (2.9%)
|
|
Asian
|
5 (4.8%) |
| Pacific Islander |
1 (1.0%)
|
Table 2: RA disease morbidities.
Key: * -subjects that were Rheumatoid Factor and/or anti-cyclic citrullinated peptide IgG positive
† – subjects with reducible or permanent articular deformities
|
|
n (%) |
| Seropositive* |
75 (71.4)
|
|
Deforming†
|
39 (37.1) |
| Erosive disease |
|
|
MRI only
|
11 (10.4) |
| X-Rays |
67 (63.8)
|
|
Extra-articular manifestations
|
|
| Nodulosis |
14 (13.3)
|
|
Interstitial Lung Disease
|
5 (4.8)
|
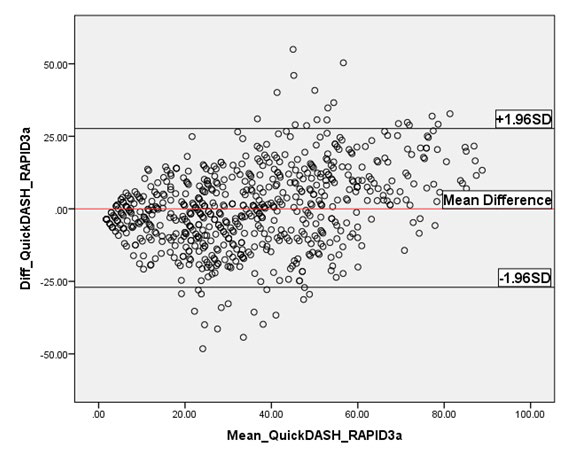
The values approximated a normal distribution with a RAPID3 average score of 11.0±6.8 and QuickDASH average score of 34.3±23.4. The QuickDASH demonstrated a very strong correlation with the RAPID3, with a Pearson correlation coefficient (‘r’) of 0.808 (p<0.0001). This is graphically depicted in Figure 1. The QuickDASH demonstrated high reliability with the RAPID3, with an interclass correlation coef><0.0001). This is graphically depicted in Figure 1. The QuickDASH demonstrated high reliability with the RAPID3, with an interclass correlation coefficient (ICC) of 0.882 (95% Confidence Interval (CI) 0.864, 0.899) (p<0.0001). Linear regression yielded the formula, F1, QuickDASH=3.595+(2.789×RAPID3). To develop the BlandAltman plot, RAPID3 unit was transformed to same unit of QuickDASH based on above formula F1. The Bland-Altman plot as shown in Figure 2 graphically demonstrates the reliability of the QuickDASH with the RAPID3. Internal consistency of the QuickDASH and RAPID3 was very good with a Cronbach alpha of 0.894.
Figure 1: RAPID3 vs. QuickDASH scatterplot. Scatterplot demonstrates strong correlation between the two questionnaires.
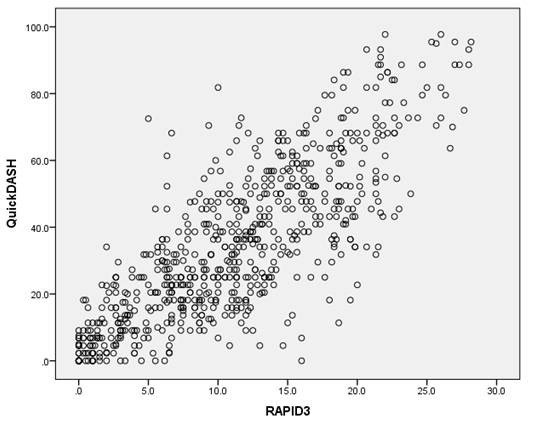
Figure 2: Bland-Altman Plot of QuickDASH vs. RAPID3 values. Plot demonstrates strong agreement between the two questionnaires.
Given the established disease activity ranges for the RAPID3, corresponding disease activity ranges were calculated for the QuickDASH using above formula F1, as shown in Table 3. The reliability of the corresponding QuickDASH disease activity ranges was high, with an ICC of 0.858 (95% CI 0.835, 0.877) (p<0.0001). Internal consistency for the QuickDASH disease activity ranges was very good with a Cronbach alpha of 0.858. The individual categorization of QuickDASH and RAPID3 values based on the established RAPID3 disease activity ranges and the calculated disease activity ranges for the QuickDASH are shown in Table 4. With the RAPID3 disease activity ranges as the standard, the calculated QuickDASH disease activity ranges had a tendency to potentially underestimate disease activity at moderate disease activity. For example, as shown in Table 4, while the RAPID3 categorized 50 subjects as having moderate disease activity, the calculated QuickDASH disease activity ranges categorized these subjects as low disease activity.
Table 3: RA Disease Activity Ranges.
Key: * – established disease activity ranges; † – proposed disease activity ranges based on equation derived through linear regression
|
QuickDASH=3.595+(2.784*RAPID3)
|
|
RAPID3* |
QuickDASH†
|
|
Remission
|
0-3.0 |
0-12.0 |
| Low Disease Activity |
3.1-6.0 |
12.1-20.0
|
|
Moderate Disease Activity
|
6.1-12.0 |
20.1-37.1 |
| High Disease Activity |
≥12.1 |
≥37.2
|
Table 4: Individual categorization of QuickDASH and RAPID3 values.
Key: LDA – Low Disease Activity; MDA – Moderate Disease Activity; HDA – High Disease Activity.
|
QuickDASH
|
| RAPID3 |
|
Remission |
LDA |
MDA |
HDA
|
|
Remission
|
90 |
11 |
11 |
0 |
| LDA |
22 |
15 |
24 |
2
|
|
MDA
|
19 |
50 |
103 |
57 |
| HDA |
7 |
6 |
58 |
223
|
Regarding secondary outcomes, the QuickDASH demonstrated a strong correlation with the Health Assessment Questionnaire, moderate correlation with the CRP, Visual Analogue Scale, and Disease Activity Score-28 (DAS28), and a weak correlation with joint counts. All of these correlations were statistically significant. The correlation of the QuickDASH with the ESR was poor and did not reach statistical significance. For comparison, correlation coefficients for the same disease activity measures or indices presented by Ochi et al6 have been included in Table 5.
Table 5: Comparison of Pearson correlation coefficients (‘r’) for QuickDASH vs other disease activity measures (with published literature)
Key: HAQ – Health Assessment Questionniare, DAS28—Disease Activity Score-28, SJC—Swollen Joint Count, TJC—Tender Joint Count, VAS—Visual Analogue Score, CRP—C-Reactive Protein, ESR—Erythrocyte Sedimentation Rate
* – not statistically significant
|
Ours
|
Ochi K et al6 |
| RAPID3 |
0.808 |
–
|
|
HAQ
|
0.843 |
0.88 |
| DAS28 |
0.582 |
0.53
|
|
SJC
|
0.204 |
– |
| TJC |
0.453 |
–
|
|
VAS
|
0.534 |
0.63 |
| CRP |
0.619 |
0.25
|
|
ESR
|
0.070* |
0.30
|
DISCUSSION
It is well established that RA disease activity relates to joint damage, and the current treatment strategy is to treat to target, specifically using one or several medications to achieve and maintain disease remission. Typical manifestations of disease activity such as swollen joints, tender joints, inflammatory markers, and pain can be measured separately using instruments or scales. They can also be measured in aggregate in a disease activity index. Of the numerous disease activity indices available, the ACR currently recommends use of one of six indices when treating to target.1 The RAPID3 questionnaire is one of these. While each possess different strengthens and weaknesses, no ideal index exists. No head to head studies exist comparing how each index performs and the Working Group which guided the process for identifying these 6 indices acknowledged that some indices excluded in their review could later be found to have adequate or superior properties in measuring RA disease activity.1 Thus, it is reasonable to explore other disease activity indices for measuring RA disease activity with the goal of refining the accuracy of how disease activity is measured. In this study, the RAPID3 questionnaire was chosen as the standardized RA disease activity index as it is fairly easy for the patient to complete, gathers only patient reported outcomes, and is easy to score. It has also been shown to be a feasible disease activity index to use in the clinical setting, the environment in which this study was conducted.7,8 This study of a heterogeneous RA population observed that the QuickDASH demonstrated high reliability and very good internal consistency when compared to the RAPID3 questionnaire. Linear regression yielded a formula which generated disease activity ranges from the RAPID3 questionnaire which again demonstrated high reliability and very good internal consistency, although it did appear that the QuickDASH ranges potentially underestimated disease activity at moderate disease activity. When compared with other measures of RA disease activity, the QuickDASH demonstrated correlation coefficients which appeared consistent with the current medical literature.
This study has several strengths. First, the RA cohort studied was diverse, with seronegative subjects comprising just under 30% of the group. An earlier study of the DASH in assessing RA disease activity evaluated solely seropositive RA subjects.4 A study in 2014 which evaluated the QuickDASH might have had a heterogeneous RA population, although the authors did not elaborate on the presence of seronegative RA patients.6 The RA population studied here also did not exclude patients with other pain conditions such as fibromyalgia, chronic pain syndrome, or musculoskeletal injuries such as rotator cuff tendinopathy. These subjects were purposefully not excluded since real world subjects often have these concomitant conditions and it is important to study a disease activity index which will be reliable and internally consistent with such concomitant disorders. Second, the QuickDASH is a validated disease activity index for upper extremity conditions which consists of only 11 questions and is easy to complete by the subject. While it has been reported to take 2 minutes to complete,2 in this clinical setting it took less than 45 seconds for subjects to complete. Subjects in this clinic routinely reported that it was easier to complete than the RAPID3 and from this study it appears that the QuickDASH could very practically be used in a busy clinical setting. Also, the QuickDASH focuses on upper extremity function, an area significantly impacted by RA. This would be reflective of the information gathered on physical examination of the RA patient, as 26 of the established 28 swollen and tender joint counts focus on upper extremity joints. Like the RAPID3, the QuickDASH is also a disease activity index that relies solely on patient reported outcomes. While this may be considered a limitation of a disease activity index, it has been noted that such indices “produce a less random and more reliable measurement of change over time.”1 Disease activity indices that measure patient reported outcomes “predict long-term outcomes better than provider joint counts and acute phase reactants.”1 The inclusion of examiner joint counts can introduce a measure that is examiner dependent, with reproducibility that may be unreliable amongst multiple observers. Thus, the QuickDASH, as a patient reported outcome disease activity index, should garner further evaluation, consistent with the recommendations of the Working Group that derived the ACR guidelines seeking indices to expand and improve the accuracy of measuring RA disease activity.1 Third, the QuickDASH correlated reasonably well with other disease activity measures and indices, yielding correlation coefficients that were statistically significant but also consistent with values published elsewhere.6 Finally, this study was the first to evaluate the validity of the QuickDASH in studying RA disease activity in English speaking patients.
This study also has several limitations which should be noted. First, the RA cohort studied was from a single center and was data gathered from one physician. Added to this, the subject size was also modest. How the QuickDASH would perform in a multi-provider group or larger medical center evaluating and treating larger cohorts of RA patients remains unknown, but it would likely perform similarly as the patient reported outcome QuickDASH should have less random and more reliable measurements of change.1 Second, the QuickDASH calculation is not intuitive. As noted, it consists of 11 questions with scoring that routinely requires a calculator. From personal experience, shortly after introducing the RAPID3 questionnaire as the disease activity index that the clinic would use, it was fairly easy to accurately score the questionnaire. Indeed, a quick glimpse of the RAPID3 questionnaire would often yield an accurate score. This ease of scoring was not apparent with the QuickDASH, except when all 11 questions were marked as zero, and in nearly every instance a calculator was needed. Third, the QuickDASH has a focused pain assessment question, one that emphases the impact that pain in the arm, hand, or shoulder has on sleeping, whereas the RAPID3 questionnaire asks how much pain the patient has experienced attributable to their condition. It is plausible to consider that the focused pain assessment question was a reason why the QuickDASH disease activity ranges underestimated disease activity when compared to the RAPID3 disease activity ranges, and thus this would limit generalized use. Fourth, educational level might influence the feasibility of self-reported questionnaires in general, although as discussed earlier the RAPID3 is composed of American College of Rheumatology (ACR) core set measures used to assess effectiveness of disease modifying therapy.3 It is sensitive to change, discriminates well between disease activity states, has remission criteria, and is feasible to perform in routine clinical cares. It is believed that this would likely be true for the QuickDASH as well, thus the reason for pursuing this clinical trial. Finally, a recent meta-analysis demonstrated that composite disease activity indices, specifically those that include swollen joint assessments, are related to radiographic progression.9 This study was not designed to assess correlation of the QuickDASH with radiographic progression, but going forward this could limit the generalized application of the QuickDASH if other disease activity indices have this property. The RAPID3, however, has data to support a correlation with radiographic progression,10 so RA disease activity indices that are patient derived still have some value in predicting radiographic progression.
CONCLUSION
In conclusion, the QuickDASH, an exclusively patient reported outcome disease activity index designed to assess the function of the upper extremities, demonstrated high reliability and very good internal consistency with a validated and endorsed RA disease activity index, the RAPID3. Compared with another published study, the QuickDASH demonstrated similar correlation with other disease activity indices and measures such as the HAQ, VAS, CRP, and joint counts. Using the establish disease activity ranges for the RAPID3, corresponding RA disease activity ranges were developed for the QuickDASH. These QuickDASH disease activity ranges appeared to underestimate disease severity when compared with those of the RAPID3, with the difference perhaps due to the absence of a pain assessment variable in the QuickDASH. Given the limitations detailed above, the data in this study should be considered empirical findings and it would be worth pursuing further validation of the QuickDASH in larger RA cohorts and in different clinical settings. If the QuickDASH remained a viable RA disease activity index, additional focus on establishing and validating changes in QuickDASH scores in relationship to disease activity change, specifically improvement, would be very informative. Also informative would be any relationship of changes in QuickDASH scores with radiographic progression in RA.







