INTRODUCTION
It is estimated that 3.5 million people living in the United States (US) are exposed to chronic Hepatitis C Virus (HCV), with many cases remaining to be diagnosed.1 The 2013 United States Preventive Services Task Force (USPSTF) recommends a onetime screening for HCV infection in adults born between 1945-1965 based on evidence indicating proven benefits of chronic HCV treatment in the reduction of all-cause mortality, cirrhosis, hepatocellular carcinoma and potential public health benefit in reducing transmission rates.2 Previous curative therapies with interferon based regimens were difficult in terms of long length of treatment and numerous side effects with only mediocre treatment outcomes.
In the current era of Direct Acting Antivirals (DAA) for chronic HCV treatment, management guidelines have been created by the Infectious Diseases Society of America (IDSA), American Association for the Study of Liver Diseases (AASLD) and the International Antiviral Society–USA (IAS–USA), to keep up with the pace with which new HCV medications are being released.3 However, the benefits of these new treatments may be offset by the limited number of patients having access to antiviral treatment, based both on insurance status and cost. It is recognized that chronic HCV patients are less likely to be insured compared to patients without this chronic illnesses. Given the recent advent of the Affordable Care Act (ACA) “ObamaCare”, the increasing number of uninsured patients will drop from 18% to 13.4% towards the end of 2015.4 The major concern about cost of treatment has led the State governments to apply restrictive access requirements with complex and burdensome associated utilization management. In addition, the insurance-driven approval of HCV medications, particularly for patients with Medicaid restricted formularies, may further affect patient care and access to treatment, thereby placing additional limitations on physician treatment options.
METHODS
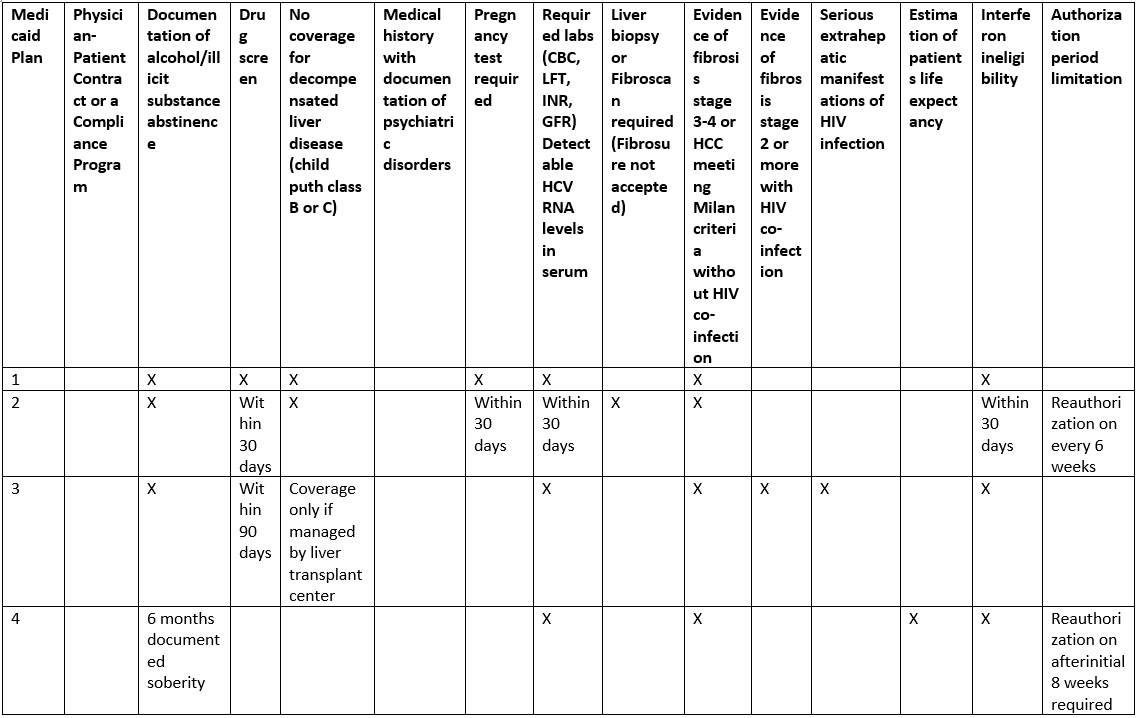
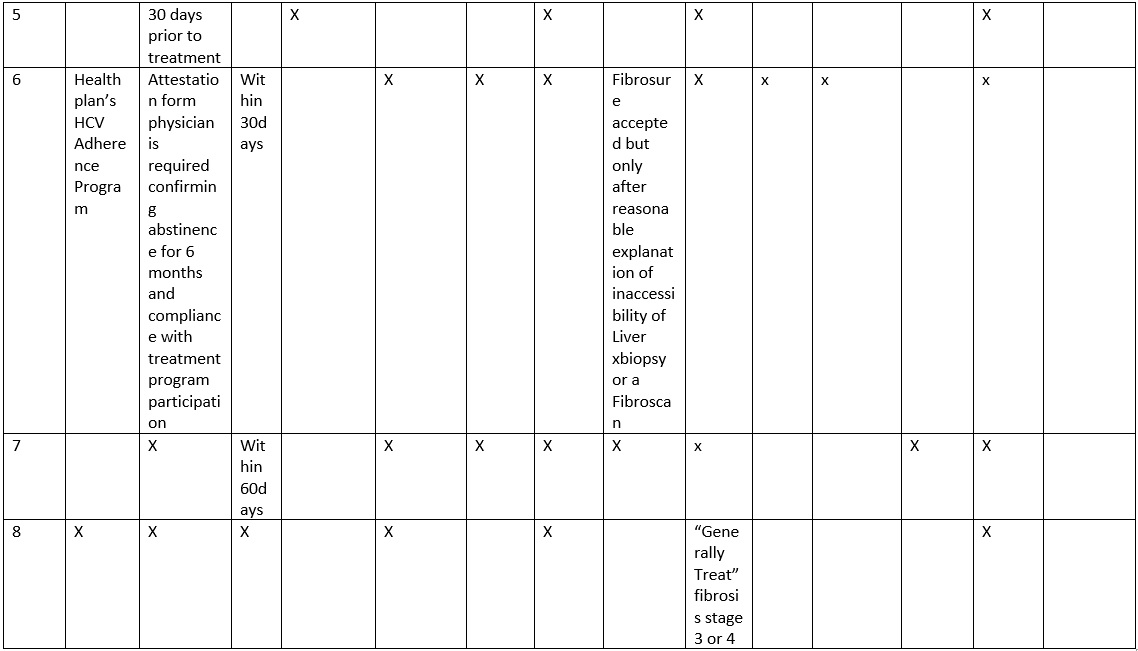
In this study, we sought to determine the HCV treatment coverage policies for DAA in Medicaid insured population. Table 1 depicts some of the salient clinical characteristics and eligibility criteria the insurance agencies are requesting for HCV medication approval. We composed a list of the Medicaid insurance carriers in the States of Ohio and Pennsylvania. Requests were then made to these insurance agencies to provide selection criteria used to determine patient candidacy for DAA therapies for HCV. In addition, HCV medication denial letters were also reviewed to further determine selection criteria.
Table 1. Comparison of eligibility criteria
FINDINGS
The Medicaid insurance coverage for DAA therapy for HCV infection across two States, Ohio and Pennsylvania, have certain similar features, but also differing requirements for medication approval (Tables 1 and 2). Inconsistency in timing of the requested blood work prior to submission of authorization is seen across insurances, ranging from 30 days to 6 months. HCVviral load tes ting is time sensitive for some insurance providers; however, no clear guideline regarding the timing of the viral load prior to initiation of treatment has been established. A complete laboratory workup to exclude other causes of liver disease is requested by most of the insurance companies. The documentation of the patient’s interferon ineligibility, NS3 Q80K polymorphism screening for genotype 1a, HIV status, TSH, uric acid, direct bilirubin, Hepatitis B serology, and ANA are required by some insurances. Interestingly, EKG is requested in patients with known cardiac disease by a few insurance providers. Abdominal imaging is requested to look for hepatocellular carcinoma in cirrhotic patients. Presence of severe or uncontrolled co-morbidities excludes a member from coverage of DAA by certain providers. Documentation of life expectancy is often requested in order to further assess the prognosis and health conditions.
Table 2: Overview of documentation and requirements.
|
Documentation
|
Requirement
|
Past treatment History
• Outcomes
• Interferon Tolerance |
Always
|
| Medical History
• Complications from HCV and liver disease
• Medical co-morbidities
• Medications |
Always
|
| Psychiatric / Social history
• Psychiatric disorders
• Substance abuse |
Always
|
| Compliance assessment and follow up
• Patient – physician contract
• Health plan’s HCV Adherence program
• Psychiatrist assessment |
Variable
|
| Life expectancy assessment |
Variable
|
| Laboratory assessment
• Testing
• Timing |
Variable
|
| Liver stage assessment
• Liver biopsy
• Fibroscan / Fibrosure
• Clinical assessment |
Variable
|
| Evaluation for contraindications to treatment |
Always
|
Clear description of psychosocial status of the patients is consistently required by all Medicaid providers across both states and is a constant reason for the delay in medications. Documentation of psychiatric disorders, past or present is required by most of the insurances, with some providers requiring notes from a psychiatrist documenting control of illness with treatment and follow up plans. In patients with known history of alcohol or substance abuse, documentation of last illicit drug and alcohol use is universally required among insurances. Along with that, most providers are requiring negative drug screens within 30 days to 6 months prior to treatment. Some insurance providers are currently offering and mandating enrollment in the HCV adherence program prior to the approval of DAA to certain members, a completed consent document indicating the adherence to HCV regimen often requested.
Medicaid programs necessitate evaluation of extent of the liver disease and fibrosis as a part of HCV treatment approval process. So far, all insurances require documentation of advanced liver disease (stage 3 and 4) prior to approval of medications. There is a high variability among insurance programs in regards to the modality used to assess liver disease. Liver biopsy is the gold standard for staging of fibrosis and it is accepted by all of the insurance providers as a documentation of the stage of liver disease. Non-invasive methods for fibrosis staging that may include Fibroscan, Elastography, Fibrosure test, or Hepascore testing are variably requested by different insurance providers. Only a few programs would accept the clinical documentation of presence or absence of advanced liver disease. The criteria of advanced liver disease is down staged by a few insurance providers in HIV/HCV co-infected patients where they qualify stage 2 or above for DAA approval in such co-infected patients.
DISCUSSION
Based on a recent review and meta-analysis, it is estimated that only 50% of the patients believed to be exposed to HCV infection are screened, 43% have access to outpatient care, 27% have HCV infection confirmed with a viral load testing, 17% undergo liver fibrosis assessment by one of the methods, 16% are prescribed treatment, and only 9% achieve Sustained Virologic Response (SVR).1 These findings are bound to change with the recent introduction of the Affordable Care Act (ACA) combined with the advent of novel DAA which have significantly higher SVR. The combined effect of availability and access of these drugs to more patients will dramatically change the healthcare and HCV landscape. ACA has improved access to healthcare coverage and offers a significantly broad access to HCV care by covering for screening, diagnosis and, in the most part, treatment of HCV. Both the USPSTF and CDC recommend one-time screening for HCV infection in the general population born from 1945 to 1965 in addition to continuing the recommendation for screening individuals at high risk for HCV.2 Under the provisions of the ACA, HCV screening is a covered service, which will improve the identification of HCV patient in the lower income individuals, as HCV is found to be more prevalent in this population.5 Many of the States are expanding Medicaid eligibility under ACA provisions. The rise in the number of individuals with insurance coverage has subsequently increased the number of patients diagnosed with HCV. Although ACA has provided significant opportunities in regard to Hepatitis C management, barriers to access of these HCV treatments still remain. Given the high cost of the medication, State Medicaid programs have substantial discretion with regard to medications coverage and related utilization management.
Review of the insurance agency criteria for DAA treatment for HCV showed apparent differential in Medicaid coverage policies in comparison to privately or Medicare insured patients. Based on the current criteria, Medicaid patients with advanced stages of fibrosis (F3- F4) are more likely to get medications approved, while patients with other insurances provide coverage regardless of the stage of fibrosis. It remains to be determined whether there will be a difference in overall HCV burden and healthcare outcomes in regards to treatment based on the stage of liver disease and the type of insurance. There is growing evidence that HCV cure with the DAA regimens has favourable public health and economic future outcomes in both late and early fibrosis stages, prior treatment history and cirrhosis.6,7
Individual Medicaid insurance providers have different criteria for the method to use for fibrosis evaluation. Liver biopsy is the gold standard for fibrosis staging and is universally accepted, while, not all insurance providers allow use and accept the results of other modalities as non-invasive tools of liver fibrosis assessment. From a clinical standpoint, liver biopsy is an invasive technique with associated morbidity. The accurate evaluation of fibrosis using liver biopsy is also complicated by sampling error and inter-observer variation in staging, particularly when inadequate sampling occurs.8,9 Liver biopsies on a largescale for staging purposes cannot be a reasonable, cost effective or a practical approach. As a result, non-invasive tools play a major role in assessment of liver fibrosis. The non-invasive approaches include the evaluation of various laboratory parameters which are included in Fibrosure/Fibrotest, or in conjunction with liver stiffness evaluation by Fibroscan, which could obviate the need for liver biopsy with over 86.7% accuracy.10 However, many medical centers still do not carry the Fibroscan or other liver stiffness measurement tools and are not able to conduct this testing with ease.
As recommended by the guidelines, the rationale of immediate and timely treatment of patients with advanced fibrosis,3 comes from numerous clinical and populations studies and is generally accepted by Medicaid.11,12 Populations at higher risk for liver disease progression, Metavir F2, co-infection populations (HIV, Hepatitis B), those with coexistent liver disease (NASH or alcoholic) and patients with extra hepatic manifestations are not regularly listed in the Medicaid initial treatment considerations criteria. Those populations should be prioritized as per expert recommendations.3 In particular, those patients with severe extra hepatic manifestations, such as Type 2 or 3 essential mixed cryoglobulinemia with end organ manifestations, have been shown to respond to immediately to treatment with appreciable benefits reduced mortality rates.13,14 These criteria are not yet adopted by most of the Medical programs.
Treatment of individuals at high risk to transmitting HCV is a major public health opportunity that may yield longterm future benefits. For example, there are no Medicaid program criteria that addresses HCV infected women of childbearing age. It is estimated the risk of HCV vertical transmission from HCV RNA positive women who are HIV negative at 5.8% and among HIV-positive women at 10.8%.15 There are discriminatory criteria with respect to persons who inject drugs (PWID) as proof of abstinence of drugs is regularly requested by Medicaid programs.16 On the other hand, the guidelines suggest that PWID should be considered for HCV treatment.3 The experience of some European countries has shown that access of PWID to HCV therapy (including DAA) and scaling up HCV treatment is feasible using an integrated multidisciplinary approach.17-19
The need for psychiatric evaluation is enforced by most of the Medicaid providers. This requirement is implemented for anyone who has used illicit drugs, or any psychotropic medication in their lifetime. The DAA do not have any significant psychiatric side effects. DAA does not induce or exacerbate anypsychiatric illnes s and thus there is no evidence behind the need for psychiatric evaluation or follow up of HCV patients while on treatment. Medicaid program use this requirement to ensure that patients will show compliance and understand the illness better if they will have a psychiatrist visit. This eligibility requirement has generated visits to psychiatrists and also delays the initiation of treatment. In addition the requirement of negative screening for drugs or alcohol is required by most of the providers as a marker or compliance and understanding of patients’ abstinence for and while on the treatment. The timing of the drug and alcohol screen varies from 30 days to 6 months among different Medicaid providers. A few health plans have formulated HCV Adherence programs, which requires enrollment by a phone call. These programs are meant to provide education and counselling in regards to compliance. Those programs are mandating the members to enroll for the approval of medications. The utility of such requirement still needs to be elucidated.
There are differences within Medicaid programs not only within a State but also between other States in regard to the required clinical documentations and testing as well as timing of these tests. Several of the blood tests which are included in the eligibility criteria do not have strict clinical evidence in regards to efficacy of DAA. Insurance companies require testing for Uric Acid, TSH or NS80K Polymorphism. These tests were needed with interferon based therapies or first generation protease inhibitors and do not have any evidence in regards to side effects or efficacy of current DAA. In addition, the Medicaid driven HCV medications, the locally approved restrictive formularies may also affect patient care and access to treatment thus limiting physician HCV treatment options and choices. Those additional requirements subsequently result in burdensome and increasingly complex pre-authorization and appeal forms especially in the rapidly evolving DAA. Though, the use of interferon is outdated in this era of DAA but still some insurance providers are requesting the documentation of reasons of interferon in-eligibility; which seems completely unnecessary as no one should be using interferon based regimens.
Certainly HCV treatment is becoming relatively easy in regards to the pill burden and medical management while on treatment. However, approval process is a daunting task, which is requiring considerable amount of time, resources and manpower. Some insurance providers are limiting the DAA to the prescriber’s specialties (hepatology, gastroenterology, infectious diseases and transplant physician). As the number of patients screened for HCV continues to increase, the provider workforce will lack the capacity to provide care for all the newly diagnosed HCV patients in need or wishing to be treated. Limiting DAA to certain prescribers may compromise public efforts to expand access to HCV treatment especially in the rural areas where the primary care clinics as the cornerstone of a “test-and-treat” approach to hepatitis C.20,21
CONCLUSION
In summary, it is indeed a very exciting time for the management of HCV infection. The convergence of the ACA with the advent of novel DAA has created new opportunities for HCV management but has also created challenges that affect various populations. As we bring increasing number of newly insured patients and these DAA into our practices, several barriers to treatment in the Medicaid population will arise. While Medicaid programs face the high cost of the emerging HCV drugs and thus implementing restrictive policies, the medical providers are trying to adjust and acquaint with those requirements. Collaborative efforts are required to further optimize and better foster access to HCV care which can only be accomplished by balancing ethical questions, evidence based data and public health goals.
CONFLICTS OF INTEREST: None







