INTRODUCTION
The conjunctiva is permanently exposed to mechanical, toxic and microbial attacks from the external environment. Its ability to adapt enables it to retain its role as a barrier, immune watchdog and mucinic secretion. Histologically, the conjunctiva consists of a non-keratinized stratified surface epithelium resting on a lamina propria separated by a basement membrane.1 Among the different cell populations, there are squamous epithelial cells and goblet cells (also called mucocytes), which ensure the production of most of the mucins, an essential constituent of the tear film. The conjunctiva fulfills a defense role, not only mechanical, but also immune thanks to the presence of immunocompetent cells, T and B-lymphocytes, and antigen-presenting cells, including Langerhans cells, which are endowed with migratory and recruitment phenomena during an attack or an inflammatory episode. Accessory lacrimal glands constantly produce some of the watery portion of the tear film.2
Mucins are high molecular weight glycoproteins and form the glycocalyx, the framework of the mucinous layer. They make this part of the tear film hydrophilic and reduce its surface tension. Without this layer, the film would not adhere and the epithelium would suffer. Mucins 1, 4 and 16 are transmembrane and produced by squamous epithelial cells, while mucins encoded by the MUC5AC gene are soluble and secreted by goblet cells. A lowlevel of mucins is associated with dry eye syndromes which can lead to partial keratinization of the conjunctiva associated with a decrease in the density of mucus cells. The mucocyte density is between 24 and 2226 cells/mm2 . 3,4 Higher densities are expected in healthy conjunctiva as well as in areas of conjunctiva not exposed to the atmosphere.5 No difference in density depending of the age was demonstrated.6 These cells disappear during ocular dryness or after long-term use of antiglaucoma eye drops containing preservatives7 and are therefore important to assess clinically. The 2α PGFs (latanoprost, travoprost, etc.) have an action on the prostaglandin F-receptors. The link between the prostaglandin F-receptor and the secretion of mucins is partially explained in particular by work on the respiratory epithelium during pathologies such as cystic fibrosis or chronic obstructive pulmonary disease.8 A study shows the role of CREB, a transcription factor activated by PGs, in increasing the formation of MUC5AC. 9 The abrogation of all downstream signaling activities via suppression of each signaling molecule along the pathway indicates that a single pathway from prostaglandin F-receptor to cAMP Response Element-Binding Protein (CREB) is responsible for inducing MUC5AC overproduction.9,10 There could be a link between prostaglandin preservative free treatment and goblet cell density and secretion.
The study of the conjunctiva is essentially based on slit lamp examination with and without vital dyes. The most reliable and widespread method for cytological analysis of the conjunctiva is the conjunctival impression.3 Nevertheless, in vivo confocal microscopy (IVCM), an emerging non-invasive technique, shows an ability to analyze the conjunctiva in vivo at a cytological scale and thus to detect mucus cells that synthesize mucins of the MUC5AC type, the main mucins tear film.11 Recently, reverse transcriptionpolymerase chain reaction (RT-PCR) studies allow the quantification of messenger ribonucleic acids (mRNAs) of mucins and class II molecules of the major histocompatibility complex such as human leucocyte antigen DR (HLA-DR) directly from conjunctival impressions and can thus provide additional functional information.12
The objectives of this study are therefore to evaluate the goblet cells density (GCD) obtained by in vivo confocal microscopy (IVCM) and conjunctival impression, secondary the clinical ocular surface parameters and finally the gene expression of MUC1, MUC5AC, HLA-DR in glaucomatous patients treated with latanoprost without preservative.
PATIENTS AND METHODS
The prospective study was conducted at the center of clinical examination at the Quinze-Vingts National Eye center over a period of 4-months in ten patients with open-angle glaucoma: four patients had never had treatment, six others had preserved PG and two had a filtering surgery. Prior to inclusion, an interview detailed the pathologies and treatments of the patients, who all signed a consent form containing the terms of the study. The study was conducted in accordance with the Declaration of Helsinki (1964) at the Center of Clinical Investigations (CIC 503) at the QuinzeVingts National Eye Center, Paris, France, with the approval of the Institutional Review Board of Saint-Antoine University Hospital (CPP-Ile de France 5, national agreement 10793). If both eyes were eligible for the study, the most symptomatic eye was chosen. Before the beginning of an hypotonic treatment with an analogue of the prostaglandin PGF2α, latanoprost in single-dose eye drops at 50 μg/ml eye drops (Monoprost®, Théa, France), a complete examination was carried out including a battery of tests: the determination of the index of suffering of the ocular surface (ocular surface disease index, OSDI), Schirmer I test, tear film break-up time (TBUT), Oxford score, measurement of intraocular pressure (IOP) and tear osmolarity, imaging of the conjunctiva by IVCM, and sampling of conjunctival impressions. Confocal microscopy and impression were made on the upper bulbar conjunctiva at 12 o’clock. Follow-up was performed at M1 and M4 after the first visit and assessed the same parameters.
IVCM was performed in all patients with Heidelberg retina Tomograph II®, Rostock Cornea Module.7 After topical anesthesia with a drop of 0.4% oxybuprocaine, the patient’s chin and forehead were positioned facing the microscope. After contact of the lens with the cornea, the patient looked down thus exposing the superior bulbar conjunctiva, the digital images were visible in real time on the control screen. The duration of the examination was approximately 10-minutes. The mucocyte density was evaluated after taking photographs using the counting software integrated into the device, by averaging the results of 5 images.
After rinsing the eye with a 0.9% NaCl solution and topical anesthesia with 0.4% oxybuprocaine, two conjunctival impressions (Supor®, Gelman, USA) were applied to the upper bulbar conjunctiva and gently removed. A fingerprint was immediately stored at -80°C to be used for RNA extraction and realtime – quantitative polymerase chain reaction (RT-qPCR) and the other was fixed in a 10% formaldehyde solution and then stained with 1% cresyl violet (Merck, Fontenay-sous-Bois, France). The mucocytes were counted by an operator in a masked manner and their mean density was determined over 5 consecutive fields at x400 magnification.
An evaluation of the transcripts was carried out by qPCR in order to compare the expression levels of the mRNAs from the cells obtained from the conjunctival imprint samples. Total RNAs were extracted from human cells using the RNA XS extraction kit (Macherey Nagel®, Düren, Germany) according to the supplier’s protocol and resin column methodology. The RNAs obtained were quantified by spectrophotometry using the Nanodrop 100 (Thermo Fisher®, MA, USA). Reverse transcription was performed on 500 ng of RNA in 20 µl using the complementary deoxyribonucleic acid (DNA) synthesis kit, ADNc (high–capacity complementary DNA (cDNA) Reverse transcription kit, Life Technologies®, CA, USA) in a thermal cycler according to the following protocol: 25 °C for 10 min, 37 °C for 60 min and 85 °C for 5-seconds. The cDNAs resulting from this enzymatic reaction were used for the PCR reaction. A real-time PCR using Taqman technology (Table 1 for the references of the probes of interest MUC1, MUC5AC, HLA-DR, and GAPDH gene of a ubiquitous enzyme serving as control) was carried out from 25 ng of cDNA subjected to the following thermal cycles: 95 °C. (10 min) and 40 cycles 95 °C. (15 sec) and 60 °C. (1 min). The reaction was performed on the 7300 real time PCR system instrument (Applied Biosystems®, MA, USA). The results and the relative quantification of the transcripts were analyzed using the threshold cycle comparison method with the following equation: 2(-ΔΔCT) or ΔΔCT=ΔCt sample -ΔCt calibrator.
| Table 1. Types and References of Probes (primers) used in PCR |
|
Taqman® probes
|
Name |
Laboratory
|
|
MUC1
|
Hs00159357_m1 |
Taqman® life technologies |
|
MUC5AC
|
Hs00873651_mH |
Taqman® life technologies |
| HLA-DR |
Hs 00219575_m1 |
Taqman® life technologies
|
| GADPH |
402869 |
Taqman® life technologies
|
The RT-PCR analysis of the conjunctival impressions was carried out on the 10 patients, taking as a calibrator the D0 moment just before the start of treatment with latanoprost single dose.
All the numerical characteristics of the patients were described according to their mean and their standard deviation. Depending on their distributions, the variables were compared using parametric or non-parametric tests. Correlations of data from repeated measurements were taken into account. All the tests were carried out bilaterally with an α risk of 5%, using the R 3.1.2 software (R Core Team 2014, Vienna, Austria).13
RESULTS
Analysis of IVCM Images and Conjunctival Impressions
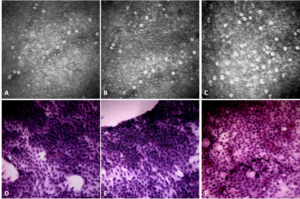
Image comparisons between IVCM and conjunctival impression collected from the same patients show excellent similarities. Indeed, conjunctival folds (in 12 patients), polynuclears (in 9 patients), mucocytes (in 30 patients), dendritic cells (in 6 patients), pseudocysts (in 6 patients), snake-like chromatin (in 6 patients) were found with both imaging methods.
In IVCM, mucocytes appeared large, oval, and hyperreflective with a hyporeflective clustered or scattered nucleus in the superficial layers of the epithelium. The mucocyte density was generally found to be higher in IVCM (Table 2, Figures 1 and 2) (153+/-96 cells/mm2 in IVCM versus 123+/-79 cells/mm2 (p<0.001) on imprints). The pseudocysts were also better visible in IVCM.
Figure 1. Conjunctival Images Comparing the IVCM and Impresion Cytology of a Patient after Therapeutic “switch” at Inclusion (A/D), at M1 (B/E) and at M4 (C/F)
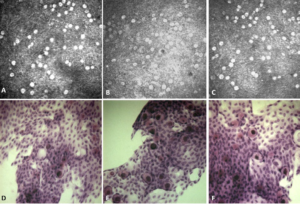
Figure 2. Conjunctival Images Comparing the IVCM and Impression Cytology of a Patient Taking de novo Treatment at Baseline (A/D), at M1 (B/E) and at M4 (C/F)
Evolution of the Clinical Parameters and GCD
On inclusion, the patients suffering from open angle glaucoma had an average age of 73+/-10-years with an average IOP of 14.8+/- 4.1 mmHg, an OSDI at 29.0+/-21.2, a TBUT at 6+/-2 sec, a Schirmer I test at 16.2+/-7.3 mm and an osmolarity at 309+/15 mOsm/L. There were 5 men for 5 women. Four patients started the latanoprost single dose in first intention (P1) while the six others introduced it following the discontinuation of bimatoprost (Lumigan®, New Delhi, India 0.01%) or travoprost (Travatan®, TX, USA) (P2).
During the follow-up of GAO patients, the mean IOP was 14.8+/-3 mmHg at D0, 13.2+/-3 mmHg at M1 and 12.9+/-3 mmHg at M4 (p=0.51). The subgroup analysis showed a tendency to pressure lowering in the P1 group (p=0.16), while the P2 group did not show any pressure change p=0.97 (Table 2).
| Table 2. Characteristics of the 10 Glaucomatous Patients at D0, M1 and M4 (P1: de novo patients, P2: previously treated patients) |
|
|
D0
|
M1 |
M4 |
p*
|
|
Mean IOP
|
P1 |
14.8+/-2.3 |
11+/-1.5 |
10.8+/-1.2 |
0.16 |
|
P2
|
14.8+/-4 |
14.7+/-3 |
14.3+/-3 |
0.97
|
| Global population |
14.8+/-3.4 |
13.2+/-2.7 |
12.9+/-3 |
0.51
|
|
OSDI
|
P1 |
23.9+/-6 |
20.3+/-7 |
19.3+/-6 |
0.66 |
|
P2
|
32.4+/-23 |
25+/-14 |
31+/-19 |
0.94
|
| Global population |
29.0+/-16 |
23.3+/-11 |
26.8+/-15 |
0.88
|
|
TBUT
|
P1 |
7+/-1 |
7.8+/-1 |
6.8+/-1 |
0.68 |
|
P2
|
4.5+/-1 |
5.7+/-1 |
6.5+/-1 |
0.11
|
|
Global population |
5.5+/-2 |
6.5+/-1 |
6.6+/-1 |
0.27
|
|
Schirmer I
|
P1 |
21+/-6 |
25+/-6 |
25+/-5 |
0.52 |
|
P2
|
13.2+/-4 |
18.7+/-7 |
20.7+/-7 |
0.25
|
| Global population |
16.2+/-5 |
21.1+/-6 |
22.2+/-6 |
0.14
|
|
Osmolarity
|
P1 |
309+/-11 |
307+/-12 |
300+/-8 |
0.44 |
|
P2
|
13.2 +/- 4 |
18.7+/-7 |
20.7+/-7 |
0.42
|
| Global population |
308.5+/-11 |
305.8+/-11 |
298.1+/-7 |
0.16
|
| *Kruskall – Wallis test |
During the follow-up of GAO patients, the mean IOP was 14.8+/-3 mmHg at D0, 13.2+/-3 mmHg at M1 and 12.9+/-3 mmHg at M4 (p=0.51). The subgroup analysis showed a tendency to pressure lowering in the P1 group (p=0.16), while the P2 group did not show any pressure change p=0.97 (Table 2).
Clinical dry eye parameters such as the ocular surface disease index (OSDI), tearfilm breakup time (TBUT), Schirmer I, and osmolarity did not vary significantly (Table 2).
The GCD was stable between D0 and M1, going from 147+/-78 cells/mm2 to 149+/-69 cells/mm2 (p=0.31) in IVCM as well as in impression (114+/-62 cells /mm2 at D0 and 120+/-56 cells/mm2 at M1, p=0.40). The GC density increased at M4 reaching 162+/-81 cells/mm2 in IVCM (p=0.02) and 134/+-61 cells/ mm2 in impression (p=0.02) (Table 3).
| Table 3. Evolution of GC Densities Measured in IVCM and Impression Cytology at D0, M1 and M4 for the 10 Patients under Hypotonic Treatment (latanoprost without preservative) |
|
GC densities
|
D0 |
M1 |
M4 |
p*
|
|
IVCM
|
147.4+/-79 |
149+/-69 |
|
0.31 |
|
162+/-81
|
0.02
|
|
Impression cytology
|
114+/-62
|
120+/-56
|
|
0.40
|
|
134/+-61 |
0.02
|
| *test of signed ranks |
Molecular Analysis of Conjunctival Epithelial Cell Transcripts
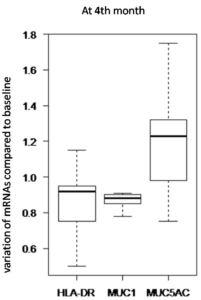
RT-PCR analysis of the conjunctival fingerprints found mean HLA-DR mRNA expression reduced by -5% at M1 (p=0.40) and by -14% at M4 (p=0.04). The mean expression of MUC1 mRNAs was reduced by -24% at M1 (p=0.01) and by -11% at M4 0.89 (p=0.06). The mean expression of MUC5AC mRNAs was increased by +23% at M1 (p=0.14) and at M4 +23% (p=0.05). In total, the expression of HLA DR and MUC1 revealed a decreasing trend over time, and on the other hand, that of MUC5AC revealed an increasing trend (Figure 3).
Figure 3. Graph Showing the Average RNA Levels of MUC5AC, MUC1 and HLA DR among the Ten Patients Treated with Monoprost® as a Function of time D0 and M4
DISCUSSION AND CONCLUSION
The IVCM is a valuable tool for examining the conjunctiva of patients and detecting goblet cells, sensitive to attacks on the ocular surface, which are clearly visible during this examination. The IVCM is a painless procedure that allows real-time analysis. It is not traumatic for the conjunctival epithelium and can therefore allow iterative examinations. The combination with the conjunctival impression is not new12,14 but no study has comprehensively compared these two methods of imaging the conjunctiva. The methods of measuring goblet cells density are still subjects of debate today.3,4 Confocal microscopy allows analysis of all the epithelial strata, whereas the impression only samples the most superficial part of the conjunctiva.3 In our study, the cell density was lower in conventional cytology as has already been reported in the literature.14 Conjunctival microcysts can be counted as goblet cell although no evidence has been provided that the microcyst is an evolved goblet cell. On filtration blebs, the morphology and immunostaining of the mucosae strongly suggested the link between the two structures.15 The detection of inflammatory cells (polymorphonuclear and dendritic cells) was similar with the two methods. Conserved or non-preserved prostaglandins are used as first-line therapy in the treatment of glaucoma. They are paracrine and autocrine signaling agents that activate many G-protein-coupled 7-transmembrane receptors (GPCRs).16 Hypotonic therapy currently offered to glaucomatous patients is based on the administration of latanoprost (Xalatan®, PA, USA and Monoprost®, Oakville, Canada), bimatoprost (Lumigan®, New Delhi, USA) or travoprost (Travatan®, TX, USA) which increase the uveoscleral flow of aqueous humor, bimatoprost increasing also trabecular flow.17
Numerous observational clinical studies have shown that hypertonic or glaucomatous patients treated complain more often of dry eye symptoms than all patients of the same age. The respective roles of active ingredients, preservatives and excipients are not fully understood. Nevertheless, the toxic and irritating effects of benzalkonium chloride (BAC), which is the most frequently used preservative, seem to be largely involved in the occurrence of these side effects.18 The instillation of preservatives on the conjunctiva can have several closely related consequences: cytotoxicity, activation of a subclinical immuno-allergic reaction, development of subconjunctival fibrosis, which can lead to progressive conjunctival scarring. The consequences on the lacrimal system (loss of goblet cells, dissolution of the lipid component of the tear film, dry eye) can be serious and lead to dry eye which is itself detrimental for the conjunctiva and the cornea. A decrease in the density of goblet cells has been observed following instillation of eye drops containing a preservative in both humans and animals.19 The first consequence of this cell loss is a change in the composition and quality of the tear film. Liang et al19 showed greater ocular surface toxicity of preserved PGs compared to preservative-free tafluprost in rabbit conjunctiva. The clinical trial refers to confocal microscopy studies carried out with timolol 0.1% without preservative20 and on tafluprost14 which showed an increase in goblet cell density with treatment duration. Our study also finds this increase in IVCM and impression cytology. The interest was to add a study of mucins by PCR from conjunctival imprints, in fact the count of goblet cells alone is not enough because cell density may therefore increase without increased secretion into the tear film.21 This study also finds an increase in MUC5AC transcripts and a decrease in MUC1 and HLA-DR transcripts. Clinical dry eye parameters such as OSDI, TBUT, Schirmer I, and osmolarity showed a slight improvement but not significantly. Our real-life study illustrates that once-daily dosing of preservative free latanoprost might result in GCD increase and could improve the ocular surface of glaucomatous patient. Overall, given the multiple functions of GCs in the ocular surface homeostasis, dedicated strategies should be adopted to preserve this cell population during the course of glaucoma.
ACKNOWLEDGMENTS
We thank the CIC of the CHNO of Quinze-Vingts and the Institute of Vision for their logistics and their technical assistance.
FUNDING
This study was not funded at all.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








