INTRODUCTION
In 2004, wild et al1 estimated that the number of people who would be living with diabetes in 2020 would be 430 million. In 2021, the International Diabetes Federation (IDF) estimated that the worldwide diabetes prevalence in adults (20-79-years) exceeded that – there were 537 million diabetic people worldwide.2 The IDF further estimates that by 2030, 543 million and by 2045, 783 million adults will be living with diabetes. It is becoming clear that the increased global prevalence of the disease may be due to population growth, life-style changes associated with urbanization, physical inactivity, obesity and aging. Unfortunately, what the current estimates also show is that while the world’s population is expected to increase by 20% by 2045, the prevalence of diabetes would be increasing at more than twice the population growth rate (46%).2
PATHOGENESIS OF DIABETES
The World Health Organization (WHO) has defined diabetes as a group of metabolic disorders characterized and identified by hyperglycemia, and the most recent position statement and Standards of Medical Care in Diabetes by the American Diabetes Association agree on the following: Four diagnostic tests for diabetes are currently recommended, including measurement of fasting plasma glucose (126 mg/dL); 2-hour (2-h) plasma glucose exceeding 200 mg/dL after a 75 g oral glucose tolerance test (OGTT); HbA1c≥6.5% and a random blood glucose exceeding 200 mg/dL in the presence of signs and symptoms of diabetes.3,4,5 Three major types of diabetes are well recognized: Type 1 diabetes, previously referred to as juvenile diabetes, occurs in about 10% in cases and may be caused by autoimmune destruction of the pancreatic β-cells, and in over 90% of cases it is T-cell mediated.3 The most common form of diabetes, is type 2 diabetes (T2DM), formerly called adult onset-diabetes, and the global estimates of diabetes prevalence actually refer to this type. Gestational diabetes occurs during pregnancy and if not managed can lead to an increased risk of developing T2DM later in both the child and mother, as well as complications during labor.
Normal blood glucose (70-105 mg/dL) is maintained by a well-coordinated balance between 3 homeostatic variables: (a) pancreatic β-cell insulin secretion in response to a glucose load, (b) insulin-mediated glucose uptake by the peripheral (primarily skeletal muscle) and splanchnic (liver and gut) tissues and (c) the ability of insulin to suppress hepatic glucose production. With increasing loss of insulin sensitivity (or increased insulin resistance), displayed as impaired insulin-mediated glucose uptake, there is induction of a compensatory chronic hyperinsulinemia (both increased fasting plasma insulin/C-peptide concentration, and increased plasma insulin/C-peptide responses to oral and intravenous glucose) in an attempt to keep blood glucose at euglycemia. When eventually the insulin production becomes inadequate to overcome the insulin resistance, the pathological state of T2DM is produced, characterized by hyperinsulinemia and hyperglycemia.6,7 When T2DM becomes firmly entrenched and blood glucose levels chronically exceed 200 mg/dL, pancreatic β-cell dysfunction may set in and pancreatic exhaustion/failure may ensue8 at which point exogenous insulin may be required. Thus, three major defects collude to produce diabetes: impaired pancreatic β-cell insulin secretion, inability to adequately suppress the hepatic glucose production, and a very significant decrease in whole body insulin sensitivity (or increased insulin resistance).
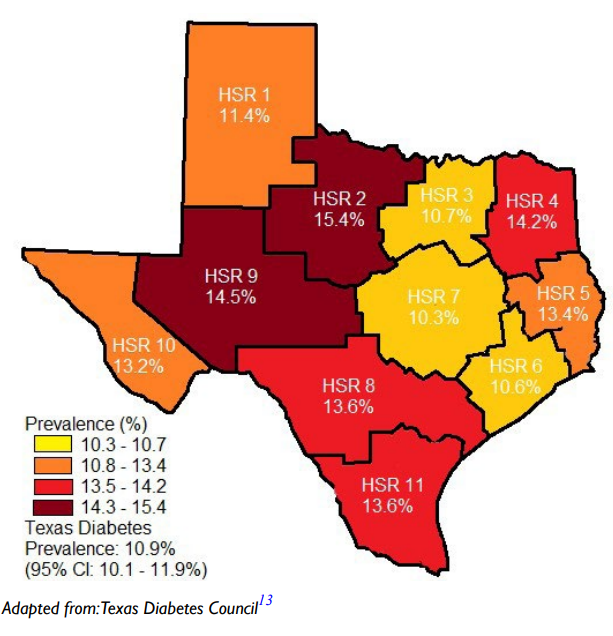
The role of the pancreatic β-cell function is very critical and any assault on its function may contribute significantly to the development of diabetes. Well-known risk factors for the development of diabetes include genetics, family history of diabetes, obesity, diet, physical inactivity the increasing global estimates which has been ascribed to aging could be due to failing pancreatic function with age.1 Approximately 9.4% of Americans (30.3 million) had diabetes in 2015 in comparison to 11.2% of Texans and the disease is disproportionately found among older adults, racial/ethnic minorities and the population with lower education. It is most common among those aged 65-75-years (about 25.9%) whereas the disease is found in only 3.1% of those aged 18-44-years.9 In several communities, the prevalence of the disease may be found in specific areas which lend credence to the suggestion that perhaps environmental factors, in addition to those well-known risk factors, must be considered as a major player. Figure 1 is a map of Texas showing the disparate prevalence of diabetes in 2015 in specific locations in the State. The Texas Department of State Health Services has divided Texas into eleven health service regions (Figure 2). The prevalence of diabetes in region 7 is at 9.8% whereas region 11 had a prevalence of 15.1%. If demographic, socioeconomic, lifestyle and genetic predisposition were held constant, it must be deduced that perhaps environmental factors may play the determining role in the disparity of type 2 diabetes (T2D) rates amongst the health regions in Texas. The health regions with higher incidence of diabetes appear to also have higher industrial activities such as fracking operations. Fracking operations and fracking wastewater disposal areas are found in greater concentrations in region 11 than region 7. These operations produce significant amounts of environmental toxicants. Excessive exposure to these toxicants may be the environmental factor responsible for a higher incidence of diabetes within certain health regions. Environmental toxicants from the fracking process have been reported in well water in the United States, and these toxicants have been shown to have endocrine disrupting activity.10,11 The 2018 Scientific Statement of the Endocrine Society lent considerable weight to the continuing acceptance that endocrine disrupting chemicals (EDCs) could play a significant role in the development of diabetes.12 When wastewater from fracking operations is improperly disposed of these toxicants make their way into the water supply thus exposing local residents to EDC’s, possibly leading to an increased incidence of diabetes in the region where fracking operations are conducted and wastewater discarded.
Figure 1. The State of Texas Showing the Prevalence of Diabetes in Specific Sections of the State in 2015
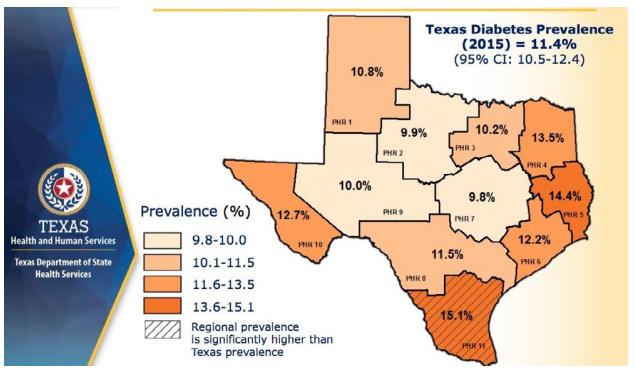
Figure 2. The state of Texas Divided into Eleven Health Service Regions According to their County with their Respective Diabetes Prevalence13
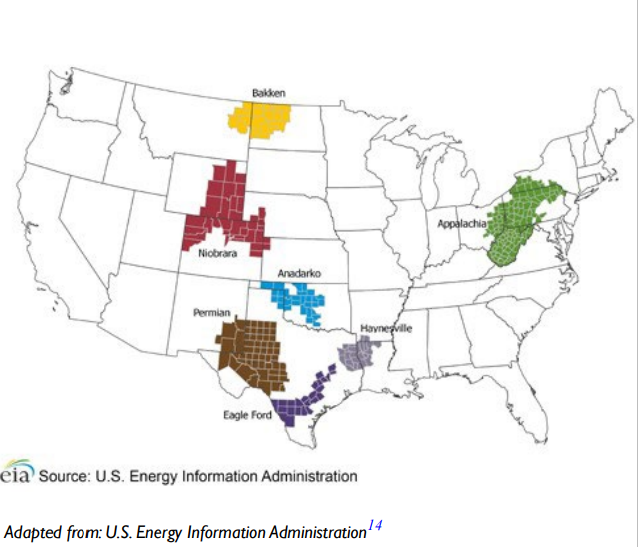
In Texas, coal mining activities occurs in health service regions 4, 5, 7, 8, and 11, while oil fracking overlaps with health service regions 1, 4, 5, 8, 9, and 11. Cotton farming in Texas takes place primarily in health service regions 1, 2, and 9. Texas contains three of the seven regions that accounted for 92% of the domestic oil production during 2011 to 2014, as seen in the Figure 3.8 The overlap of these high diabetes prevalence rates and areas of human activity may lead to the release of environmental toxicants and may be a contributor to the rising diabetes epidemic.
Figure 3. Map of the United States Showing the Main Three Regions of Oil Production in Texas Highlighted
CANDIDATE ENVIRONMENTAL TOXICANTS
Studies from Taiwan, Bangladesh, and Mexico have reported high-levels of arsenic (exceeding 100 μg/L) in drinking water and those levels have been linked to increased diabetes prevalence in those areas.15,16,17 It has been suggested that inorganic arsenic (iA) and possibly its metabolites, found in the contaminated drinking water, may be able to interfere with either insulin stimulating signal transduction pathways or with critical steps in glucose metabolism. This may ultimately interfere with the transport of glucose into the cell, allowing excessive extracellular glucose to accumulate which may lead to development of diabetes. The iA form of arsenic is usually found in drinking water from natural mineral deposits and in food produce. Millions of people worldwide are exposed to arsenic contaminated water and even in the United States, approximately 13 million individuals are estimated to live in areas where the iA level apparently exceeds the standard level, 10 μg/L.18 Arsenic has therefore being labeled as diabetogenic, interfering several aspects of glucose metabolism, including pancreatic β-cell insulin secretion.
Another environmental toxicant implicated is cadmium, an elemental pollutant which spreads through the environment as a result of industrial and farming activities. United States and the European Union have set the average human daily consumption at 0.3 μg/kg/d.19,20,21 Considerably higher daily cadmium intake was found in eastern Asian countries, such as China, Japan, Vietnam, and Thailand.22 In a mouse model, it has been shown that exposure to cadmium caused pancreatic β-cell dysfunction and death, both in vitro and in vivo.23
Environmental chemicals better known as persistent organic pollutants (POPs) are also implicated in the development of diabetes. One example is Dioxin, which has been shown to cause various adverse health effects in humans such as skin lesions, altered liver function, and impaired immune system.24,25 Dioxins are a family of chemicals that includes three different groups: polychlorinated dibenzo-p dioxins, polychlorinated dibenzofurans, and certain polychlorinated biphenyls (PCBs). Common sources of dioxins include waste and fuel combustion, refining processes, and chemical manufacturing. Elevated dioxin levels within the blood may result from atmospheric exposure, or intake of water and food that may contain dioxins. These compounds accumulate in the human body and are stored predominantly in fatty tissues.25 A 2008 cross-sectional study in Japan on 1374 individuals who were exposed to ‘naturally’ occurring environmental toxicants showed that Japan may have exposure levels that is about 10 times the levels in other industrialized countries. Elevated HbA1c (>6.1%) levels was observed to correlate with elevated dioxin serum concentrations.26 The mechanism, although not properly understood suggests dioxins may increase insulin resistance as shown in Vietnam veterans.27
CONCLUSION
In conclusion, a lot is already known about the major host risk factors that contribute to the development of diabetes, but the rapidly increasing global prevalence of the disease in certain parts of the world with known with environmental pollutants, calls for examination of other important factors outside the host that might contribute to the disease process. To account for the global rise of diabetes, several pollutants have been implicated, as is the varied proposed mechanisms including increased oxidative stress–the generation of reactive oxygen species, which overwhelm the cells intrinsic antioxidant capacities.28 Several metals, including arsenic, cadmium, mercury and lead have been shown to cause oxidative damage.29 To best understand how this affects diabetes in particular, a concerted effort must be made to classify how these compounds may directly increase hepatic glucose production, decrease whole body insulin sensitivity or impair pancreatic β-cell function, the three major conditions that collude to produce diabetes.7 Isolating and classifying the insulting compounds along these three lines will greatly enhance our understanding, which will prompt us to limit human activity in certain geographical areas or find alternative ways for certain industrial activities or better protection from exposure.








