INTRODUCTION
In recent years, it has becoming increasingly accepted that food supplements have beneficial effects on human health. Their role in correcting nutritional deficiencies, maintaining an adequate intake of certain nutrients, and in supporting specific physiological functions is extensively accepted among the scientific community. The increasing interest in food supplement use and their popularity also gained the attention of the council for responsible nutrition (CRN) and pollsters. An online survey conducted online by Ipsos Public Affairs for the CRN, found that 76% of adults in the United States take food supplements.1 Interestingly, the same percentage of respondents perceives the food supplement industry as trustworthy with an increasing trend by 3% from the previous year. This evidence rapidly gained the attention of both the industry and the consumers and the role of food supplements in supporting specific physiological functions was associated with the impact of nutritional deficiencies and their effect on skin, hair, and nails. Despite the relationship between nutrition and skin, hair, and nails has been difficult to substantiate in dermatology, there is reason to believe that a healthy diet can significantly contribute to their appearance; therefore, natural bioactive compounds administered by the oral route can represent an effective way to improve both hair and nails conditions.2,3 Some food supplements are well known to be effective due to the historical use and word of mouth while others were demonstrated to be effective in extensive studies on their efficacy. However, when it comes to the claims accompanying skin, hair, and nails food supplements, most of the time, they are not supported by evidence-based-science.2,4
In a previous study, we demonstrated the effect of LCystine amino acid in improving hair and nail conditions.5 Nowadays, due to the increasing evidence that protein-based dietary supplements can be effective in reducing hair loss6 and improving nails conditions,7 we extended our previous research to nutrients obtained from poultry feathers which composition is close to the human keratin and also highly bioavailable for the organism. As a matter of fact, the use of animal co-products in food supplementation represents an effective way to make them value-added products and to decrease their impact on the environmental pollution.8
The reason behind our interest in hair and nails was related to their impact on the subject’s self-esteem and wellness. Hair and nails are imbued with more social and psychological significance than with biological importance. Although most of the hair and nails altered conditions are not life-threatening, they have a significant impact on the affected subject’s wellness and their social interactions. Both for men and women, hair is a physical attribute expressing their individuality and attractiveness.9 The expression “bad hair day” or “tearing my hair” are emblematic and testimony to the psychological importance of hair. In 2001, Williamson and collaborators reported a decrease of the quality of life and restricted social contacts, correlated with symptoms of depression, in subjects of both sexes affected by various forms of hair loss10 with a more severe psychological impact among women than in men.11,12 Among all the hair loss disorders, we focused our attention on acute telogen effluvium (aTE). The term “telogen effluvium” (TE) was proposed to differentiate a dermatological condition characterized by diffuse, non-scarring shedding of hair from excessive shedding of normal club hair.13 Telogen effluvium is one of the most common causes of alopecia. Most of the cases of TE are subclinical therefore its real incidence is not known14 even if it is considered to be quite a common condition with a large percentage of adults experiencing an episode of TE at some point of their life.15 Despite there the absence of clear data on the TE incidence it is well known that it can occur both in men and women without any racial predilection.14,15 Women take hair shedding problem more seriously than men and are likely over-represented in seeking medical treatment.16–19
Like hair also nails are a physical attribute with a significant impact on the individual psychology and attractiveness. The expression “bite my nail” and nail-biting are emblematic behaviors in such situation in which a subject is nervous, frustrated or stressed. The impact of nails disorders on the quality of life was reported by Belyayeva and collaborators in a study including 1063 patients with nails disorders. The authors found, among others, that the quality of life was statistically significantly more affected in patients with nails structure abnormalities. It is interesting to note that the authors concluded that the impact on the quality of life is more related to the appearance of the nails than the severity of the disorder.20 Among all the nails disorders we focused our attention on the “brittle nail syndrome (BNS)”. The clinical signs of BNS include horizontal splits within the nail plate (onychoschizia) and increased longitudinal ridging or splitting (onychorrhexis): the impairment of intercellular adhesive factors of the nail plate is expressed as onychoschizia; while the involvement of the nail matrix is expressed as onychorrhexis.21 The incidence of BNS was estimated by 20% of the population, especially women over 50 years of age, with fingernail fragility being more prevalent than toenail fragility.22
MATERIALS AND METHODS
This was a single-site, randomized, double-blind, placebo- and benchmark-controlled study. Before any study-related procedures take place, the subjects were informed on study risks and benefits. Informed subjects were then asked to sign an informed consent form. The study protocol and the informed consent form were approved by the “Independent Ethical Committee for Non-Pharmacological Clinical trials” during its meeting on December 12th, 2016. The study duration was 90 days. Subjects attended clinic visits at baseline and after 45 and 90 days product intake. Each subject took 1000 mg/day of a natural keratin hydrolysate (Kera-Diet® or benchmark product) or a placebo for 90 days. Primary endpoints were the measurement of anagen/telogen hair and the nail growth speed. The study flow and the schedule of assessments chart is reported in Figure 1.
Figure 1. Study Flow and Schedule of Assessment Chart
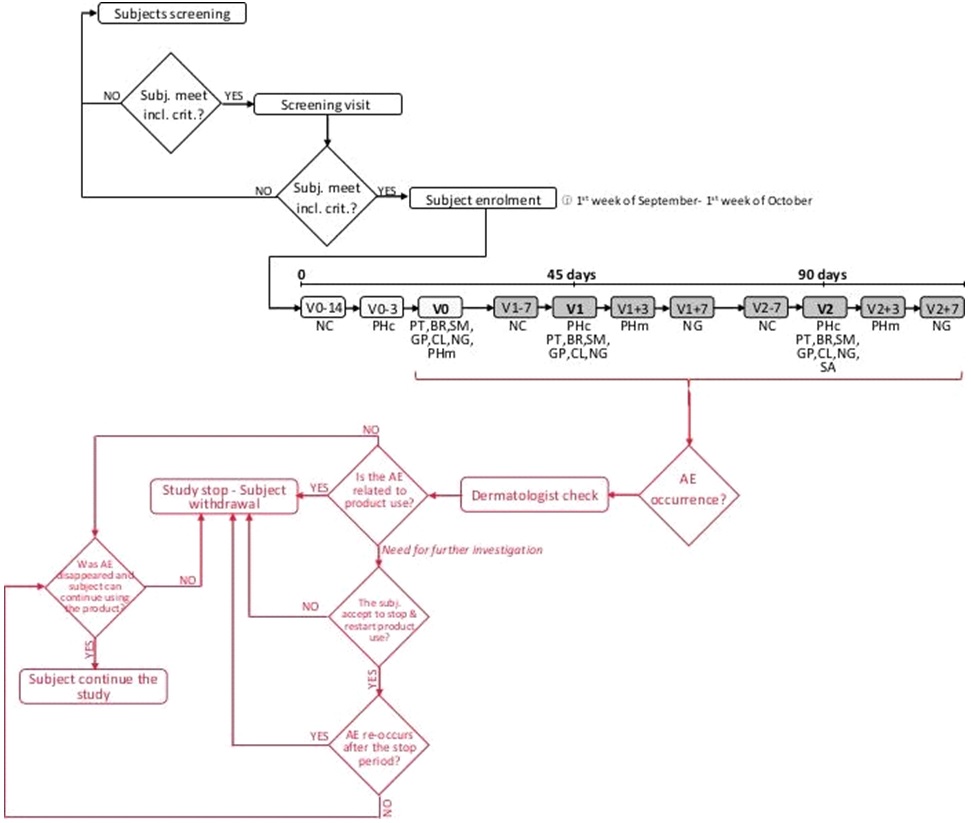
The study took place at Complife Italia dermatological facilities in San Martino Siccomario (PV), Italy. Complife Italia is an independent testing laboratory for in vitro and in vivo safety and efficacy assessment of cosmetics, food supplements, and medical devices.
Subjects
Participants (N=60, n=20 subjects per each treatment arm) were enrolled at Complife Italia by a board-certified dermatologist. Inclusion criteria were general good health, age between 30 and 60 years old, ongoing aTE and BNS (not pathological condition), no alimentary/eating disorders (i.e. bulimia, psychogenic eating disorders, etc.), and known history of metabolic syndrome. Non-inclusion criteria were pharmacological treatments known to interfere with the test product or masking the testing product effects (e.g. anti-mitotic, cytotoxic drugs other than antineoplastic, retinoids, androgens, anti-androgens, anti-epileptic agents, interferon-alpha), simultaneous participation in a similar study, history for radiotherapy or chemotherapy treatments, scalp surgery (e.g. hair transplantation), pregnancy (included intention to become pregnant) and lactation, use of food supplements having an influence on hair loss growth and on nail plate, systemic or local treatments for androgenetic alopecia (Minoxidil, Aminexil, Finasteride, Dutasteride, cosmetic solution or capsules with vitamin B, zinc, caffeine), excessive or fluctuating hair shedding for more than 6 months. Before study initiation subjects were asked to cut their nails 7 days before and after each checkpoint. Subjects were asked to refrain from hair dyeing and to cut their hair during all the study period.
The number of subjects to be included in the study panel was calculated using a statistical software (PASS 11, Version 11.08 for Microsoft Windows). A sample size of 20 subjects per group was necessary given an anticipated dropout rate of 20%.
Intervention
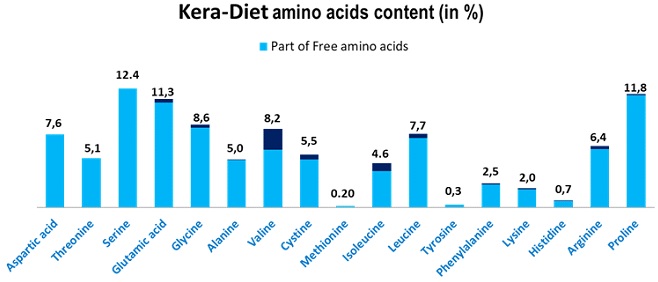
All subjects were given a three-month supply of the test product (Kera-Diet®, BCF Life Sciences, Boisel, 56140 Pleucadeuc, France), benchmark product or placebo product. The way of use was as follows: 2 capsules at breakfast and two capsules at dinner. The ingredient list of each product is reported in Table 1. The total active (Kera-diet® or benchmark keratin) intake per day was 1000 mg. Kera-diet® has an amino acid profile similar to human hair and contains a high-level of free amino acids (>92 %) (Figure 2). The active (KD), Benchmark (BE) and placebo (PL) products distribution was stratified using biased coin Efron’s algorithm with a 1:1:1 allocation ratio (PASS 11, version 11.0.8; PASS, LLC. Kaysville, UT, USA). The allocation sequence was concealed from the insite study director in sequentially numbered, opaque, and sealed envelopes, reporting the unblinded treatment allocation (based on subject entry number in the study). The A4 sheet reporting the unblinded treatment was folded to make the envelope impermeable to intense light. After acceptance of the subject in the study, the appropriate numbered envelope was opened. The test products were dispensed by a technician according to the masked randomization card contained in each envelope. The investigator and its collaborators who obtained outcome measurements were not informed on the product group assignment. Neither the investigator (and her collaborators) nor the study subjects knew who was getting the active or the benchmark products and who was getting the placebo products. The active, benchmark and placebo products were in capsule form and identical in appearance. They were prepacked in blisters and consecutively numbered for each subject according to the randomization schedule. Each subject was assigned an order number and received the capsules in the corresponding prepacked blister.
| Table 1. Capsule Composition. Quantities are Reported in mg. |
|
KD 250 mg
|
BE 250 mg
|
PL
|
| Ingredients |
| Maltodextrin |
250
|
250
|
524.3
|
| Kera-Diet® |
250
|
—
|
—
|
| Other keratin (benchmark ingredient) |
—
|
250
|
—
|
| Magnesium stearate |
14
|
14
|
14
|
| Zinc sulphate heptahydrate (22% Zn) |
11.36
|
11.36
|
—
|
| Silice |
5
|
5
|
5
|
| Vitamin B3 (nicotinamide) |
4.5
|
4.5
|
—
|
| Vitamin B5 (D-Calcium pantothenate) |
3.72
|
3.72
|
—
|
| Dry extract of aerial part of Equisetum Arvense |
2.5
|
2.5
|
—
|
| Copper sulphate pentahydrate |
1.48
|
1.48
|
—
|
| Vitamin B6 (pyridoxine hydrochloride) |
0.6326
|
0.6326
|
—
|
| Vitamin B8 (biotin) |
0.15
|
0.15
|
—
|
| KD: Kera-Diet®; BE: Benchmark product; PL: Placebo. |
Figure 2. Kera-Diet® Aminoacidic Composition
Hair Loss Measurement/Assessment
Hair loss related parameters were measured/investigated by both phototrichogram and pull testing.
Phototrichogram: A target area of the scalp (mid vertex) was clipped using a hair trimmer (Moser edition, WAHL Italia, Bologna, Italy). Short-clipped hair were removed using an adhesive tape. Three days (72±2-hours) after hair clipping, hair was dyed using a commercially available hair dye (Goldwell topchic, black 2N, Darmstadt, Germany with Rondo 6% CrèmeOxyd, Coiffeur, Cologne, Germany). After 15-minutes the colored area was thoroughly cleaned with an alcoholic solution (Kodan® Spray, Schülke & Mayr, Vienna, Austria) and digital images were taken using a DermoGenius camera (DermoScan GmbH, D-93055 Regensburg, Germany). Pictures were then analyzed by TrichoScan® analysis (software version 2.3). At follow-up visit, the target area was identified using a homemade repositioning device instead of a tattoo landmarking.
Pull testing: The hair pull test was carried out by the dermatologist. The procedure consists in gently pulling of a little clump (20-60 hair) of hair. Pull testing was repeated in three different scalp areas (frontal, temporal, and occipital region). If more than three hair per each area (or more than ten hair over the three areas) were removed, the pull test was considered as positive and suggestive of telogen effluvium.
Digital Pictures and Image Analysis
Digital photographic pictures of the hair (the vertex and frontal level) and the nail plate were taken under standard lighting conditions using a professional digital reflex camera NIKON D300/D600 digital (Nital S.p.A., 10024 Moncalieri, To, Italy) camera equipped with a macro-objective (AF-S Micro NIKKOR 60 mm f/2.8G ED), an independent flash system (Kit R1C1) and with cross- and parallel- polarized filters.
Nail plate growth was measured, using a morphometric image analysis technique, as the difference between the total nail length after cutting and the total nail length after 14 days from cutting.
Clinical Scoring
The overall hair volume was scored by the dermatologist on the pictures taken before and after treatment using a seven-point rating scale as follows: +3 greatly increased hair volume; +2 moderately increased hair volume; +1 slightly increased hair volume; 0 no change in hair volume; -1 slightly decreased hair volume; -2 moderately decreased hair volume; -3 greatly decreased hair volume.23,24
Hair and Nails Brightness
Hair and nails brightness was measured using a spectrophotometer/colorimeter CM-700D (Konica- Minolta, 20092 Cinisello Balsamo, MI, Italy). The measured parameter was the 8° gloss (specularly reflected light).
Self-Assessment Questionnaire
Subjects were asked to reply to the questions of a self-assessment questionnaire.
Statistical Analysis
Statistical analysis was performed using NCSS 10 (version 10.0.12 for Windows; NCCS, LLC. Kaysville, UT, USA) running on Windows Server 2008 R2 Standard SP1 64-bit edition (Microsoft, USA). Data normality was checked using Shapiro-Wilk W normality test and data shape. Intragroup (vs. baseline) statistical analysis was carried out using repeated measures analysis of variance (RM- ANOVA) followed by Tukey-Kramer post-test. Intergroup (between treatments) statistical analysis was carried out using RM-ANOVA followed by tests for two-factor interactions. A p-value<0.05 was considered statistically significant. Statistical analysis output was reported as follows: *p<0.05, **p<0.01, and ***p<0.001.
RESULTS
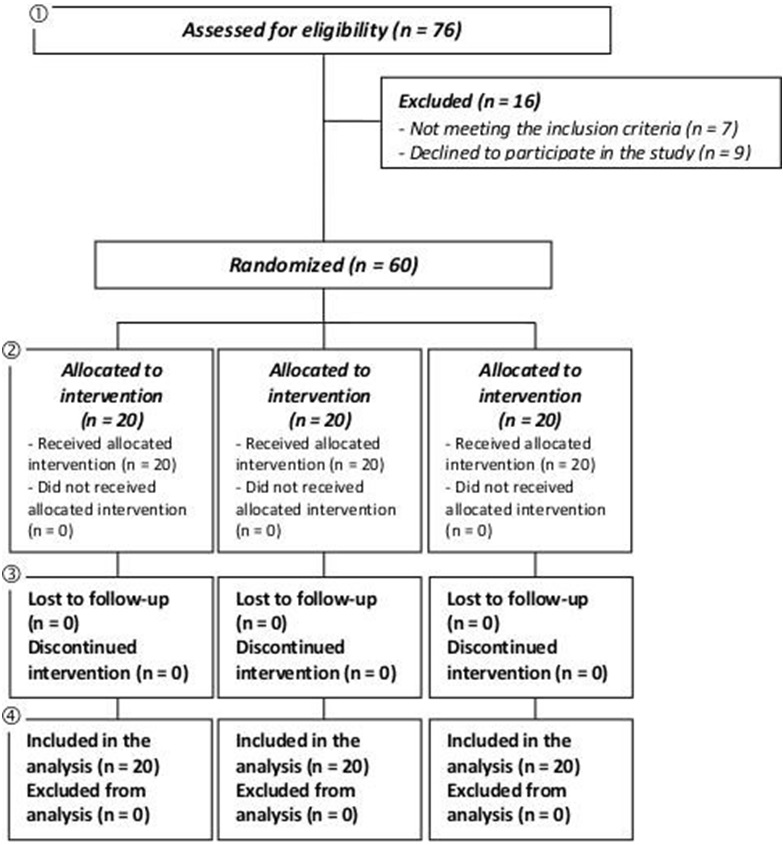
The study was conducted between February and July 2017. A total of 60 female subjects were successfully randomized (Figure 3). The population was Caucasian. Demographic and baseline characteristics (Table 2) were similar across treatment arms, indicating unbiased randomization and the absence of covariates. Subjects attended clinic visits at the time of randomization (baseline) and after 45 and 90 days of product use. Data analysis was intention-to-treat and involved all subjects who were randomly assigned. Subjects’ compliance to treatment was assessed by product accountability, as follows: at each visit, the expected amount of consumed capsule was compared with the amount dispensed minus the amount the subject returned. No major deviations were observed in the treatment regimen. All subjects were included in the safety analysis data set. All the tested products were well tolerated. No adverse reactions occurred during the study period. A statistically significant increase in hair density was observed both in the KD and BE treatment groups (Table 3a). The hair density (number/cm2) was significantly increased in the KD treatment group by 2.1±0.7 and by 13.3±1.7, after 45 and 90 days, respectively (p<0.001). A similar efficacy profile was seen for the BE treatment group where hair density was increased by 2.2±0.8 and by 9.6±1.3, after 45 and 90 days, respectively (p<0.001). The variation of hair density was not statistically significant in the placebo group (p<0.001). A similar efficacy profile was seen for the BE treatment group where hair density was increased by 2.2±0.8 and by 9.6±1.3, after 45 and 90 days, respectively (p<0.001). The variation of hair density was not statistically significant in the placebo group (p>0.05). Both KD and BE hair density variation was statistically significant when compared to the placebo group (p<0.05).
Figure 3. CONSORT 2010 Flow Diagram
| Table 2. Demographic and Baseline Characteristics |
|
KD
|
BE
|
PL
|
| Sex |
| Female |
100%
|
100%
|
100%
|
| Phototricogram |
| %telogen |
21.3±0.6
|
20.7±0.7
|
21.0±0.8
|
| %anagen |
78.7±0.6
|
79.3±0.7
|
79.0±0.8
|
| Hair density (hair no/cm2) |
192.7±6.7
|
195.7±7.1
|
193.2±7.4
|
| Pull test |
12.3±0.3
|
12.6±0.4
|
12.3±0.3
|
| Hair radiance |
3.36±0.41
|
3.31±0.38
|
3.33±0.35
|
| Nail growth rate (mm/14 days) |
1.24±0.07
|
1.28±0.06
|
1.26±0.06
|
| Nail brightness |
7.32±0.72
|
7.41±0.50
|
7.28±0.51
|
| Data are Means±SE. KD: Kera-Diet®, BE: Benchmark, PL: Placebo. |
A statistically significant increase of anagen hair was observed both in the KD and BE treatment groups (Table 3b). The percentage anagen hair was increased in the KD treatment group by 3.6±0.6% and by 9.1±1.0%, after 45 and 90 days, respectively (p<0.001). A similar efficacy profile was seen for the BE treatment group where the percentage of anagen hair was increased by 3.9±0.4% and by 8.6±0.9% after 45 and 90 days, respectively (p<0.001). The variation of percentage of anagen hair was not statistically significant in the placebo group (p<0.05). Both KD and BE variation in the percentage of anagen hair was statistically significant when compared to the placebo group (p<0.05). The same results were obtained for the percentage of telogen hair variation (Table 3c). A statistically significant decrease of the number of pulled hair was observed both in the KD and BE treatment groups (Table 3d). The number of pulled hair was decreased in the KD treatment group by 26.5±3.7% and by 32.7±3.6%, after 45 and 90 days, respectively (p<0.001). A similar efficacy profile was seen for the BE treatment group where the number of pulled hair was decreased by 22.9±2.2% and by 33.5±2.7% after 45 and 90 days, respectively (p<0.001). A statistically significant decrease of the number of pulled hair (-10.6±2.6%) was seen in the placebo group after 90 days of product use. Both KD and BE variation in the number of pulled hair was statistically significant when compared to the placebo group (p<0.05). Interestingly, according to the pull testing scoring system (number of pulled hair≤10 hair), the diagnosis of excessive hair shedding due to aTE was negative both for KD and BE treatment groups. Figure 4 shows the macroscopic effect of the product in decreasing hair loss. Hair volume and nail conditions were improved both in the KD and BE treatment groups. The subjects showing an improvement of hair volume in the KD treatment group were 40% and 65%, after 45 and 90 days respectively. A simlar efficacy profile was seen for the BE treatment group where p<0.05). The same results were obtained for the percentage of telogen hair variation (Table 3c).
| Table 3: Phototricogram and Pull Testing Results |
|
n
|
Mean±SE
|
Min÷Max
|
|
KD
|
BE
|
PL
|
KD
|
BE
|
PL
|
| a) Hair density (no./cm2) |
| Day 0 |
20
|
192.7±6.7a
|
195.7±7.1a
|
193.2±7.4a
|
155.0÷250.6
|
152.7÷252.3
|
138.3÷246.2
|
| Day 45 |
20
|
194.7±6.7b
|
197.9±7.1b
|
194.0±7.1a
|
160.2÷251.2
|
160.1÷258.5
|
142.2÷248.4
|
| Day 90 |
20
|
205.9±6.4c
|
205.4±6.7c
|
195.0±7.2a
|
172.3÷266.3
|
165.2÷268.3
|
145.7÷256.3
|
| D45-D0 |
20
|
2.1±0.7†
|
2.2±0.8†
|
0.8±0.8
|
-3.2÷7.2
|
-6.2÷12.9
|
-5.5÷5.9
|
| D90-D0 |
20
|
13.3±1.7†
|
9.6±1.3†
|
1.8±0.9
|
-6.8÷21.2
|
-3.4÷40.2
|
-2.8÷10.1
|
| b) Anagen (%) |
| Day 0 |
20
|
78.7±0.6a
|
79.3±0.7a
|
79.0±0.8a
|
72.8÷82.5
|
71.1÷84.5
|
71.6÷83.7
|
| Day 45 |
20
|
82.3±0.7b
|
83.2±0.7b
|
78.6±0.7a
|
74.9÷89.8
|
77.9÷88.8
|
71.1÷84.5
|
| Day 90 |
20
|
87.7±0.6c
|
88.0±0.6c
|
82.2±0.7b
|
84.0÷91.9
|
83.6÷91.9
|
74.5÷87.6
|
| D45-D0 |
20
|
3.6±0.6†
|
3.9±0.4†
|
-0.4±0.5
|
-0.3÷11.1
|
-0.2÷11.2
|
-5.5÷3.2
|
| D90-D0 |
20
|
9.1±1.0†
|
8.6±0.9†
|
3.2±0.9
|
-11.1÷0.3
|
6.0÷18.2
|
-1.9÷12.3
|
| c) Telogen hair (%) |
| Day 0 |
20
|
21.3±0.6c
|
20.7±0.7c
|
21.0±0.8c
|
17.5÷27.2
|
15.5÷28.9
|
16.3÷28.4
|
| Day 45 |
20
|
17.8±0.7b
|
16.8±0.7b
|
21.4±0.7c
|
10.2÷25.1
|
11.2÷22.1
|
15.5÷28.9
|
| Day 90 |
20
|
12.3±0.6c
|
12.0±0.6c
|
17.9±0.7b
|
8.1÷16.0
|
8.1÷16.4
|
12.4÷25.5
|
| D45-D0 |
20
|
-3.6±0.6
|
-3.9±0.4
|
0.4±0.5
|
-11.1÷0.3
|
-11.2÷0.2
|
-18.2÷-6.0
|
| D90-D0 |
20
|
-9.1±1.0†
|
-8.6±0.9†
|
-3.2±0.9
|
-18.0÷-1.5
|
-5.5÷3.2
|
-12.3÷1.9
|
| d) Pulled hair (hair no.) |
| Day 0 |
20
|
12.3±0.3c
|
12.6±0.4c
|
12.3±0.3c
|
11÷16
|
11÷17
|
11÷16
|
| Day 45 |
20
|
8.9±0.4b
|
9.7±0.5b
|
12.4±0.5c
|
4÷11
|
7÷16
|
10÷20
|
| Day 90 |
20
|
8.1±0.4a
|
8.3±0.4a
|
11.0±0.5b
|
3÷11
|
5÷12
|
8÷16
|
| D45-D0 |
20
|
-3.4±0.6†
|
-2.9±0.3†
|
0.2±0.4
|
-11÷0
|
-5÷-1
|
4÷-2
|
| D90-D0 |
20
|
-4.2±0.6†
|
-4.3±0.4†
|
-1.3±0.3
|
-12÷-2
|
-9÷-2
|
1÷-3
|
| a) Hair density. b) Anagen hair. c) Telogen hair. d) Pull test results. Significantly different from D0: a<b<c, p<0.05. RM-ANOVA followed by Tukey- Kramer post-test. †Significantly different (p<0.05) from Placebo. RM-ANOVA followed by tests for two-factor interactions. |
Figure 4. Global Photography Assessment
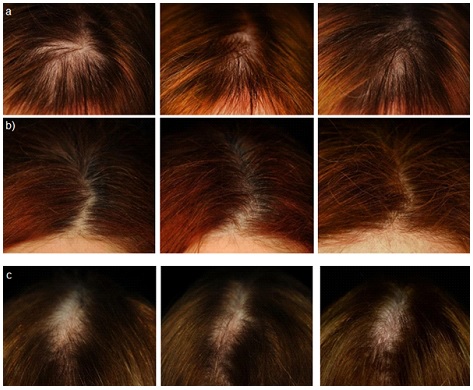
a) KD group b) BE group c) PL group
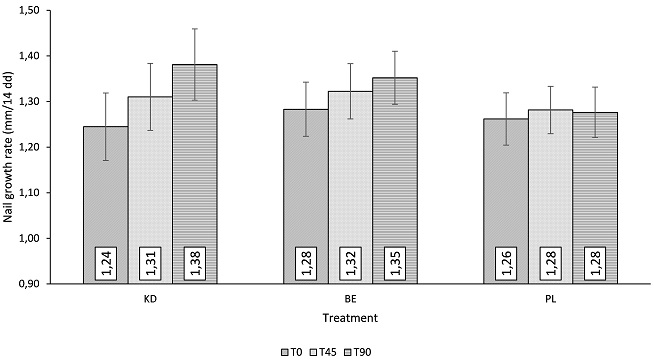
A statistically significant decrease of the number of pulled hair was observed both in the KD and BE treatment groups (Table 3d). The number of pulled hair was decreased in the KD treatment group by 26.5±3.7% and by 32.7±3.6%, after 45 and 90 days, respectively (p<0.001). A similar efficacy profile was seen for the BE treatment group where the number of pulled hair was decreased by 22.9±2.2% and by 33.5±2.7% after 45 and 90 days, respectively (p<0.001). A statistically significant decrease of the number of pulled hair (-10.6±2.6%) was seen in the placebo group after 90 days of product use. Both KD and BE variation in the number of pulled hair was statistically significant when compared to the placebo group (p<0.05). Interestingly, according to the pull testing scoring system (number of pulled hair≤10 hair), the diagnosis of excessive hair shedding due to aTE was negative both for KD and BE treatment groups. Figure 4 shows the macroscopic effect of the product in decreasing hair loss. Hair volume and nail conditions were improved both in the KD and BE treatment groups. The subjects showing an improvement of hair volume in the KD treatment group were 40% and 65%, after 45 and 90 days respectively. A simlar efficacy profile was seen for the BE treatment group where the subjects improved were 30% and 55%, after 45 and 90 days. The improvement was statistically significant for both the KD and BE treatment groups when compared to the placebo group (5% and 10% of the subjects, after 45 and 90 days). The subjects showing an improvement of nail conditions in the KD treatment group were 30% and 60%, after 45 and 90 days; while the percentage of subjects improved in the BE treatment group was 30% and 55%, after 45 and 90 days. The improvement was statistically significant for both the KD and BE treatment groups when compared to the placebo group (15% and 20% of the subjects, after 45 and 90 days). Both the hair and nail brightness were statistically significant improved (Table 4) in the KD and BE treatment groups. Hair brightness in the KD treatment group was improved by 23.8% and 57.0%, after 45 and 90 days. A similar efficacy profile was seen for the BE treatment group where hair brightness was improved by 13.7% and 41.9%, after 45 and 90 days. The improvement of hair brightness, both in the KD and the BE treatment group was statistically significant when compared to the placebo group (p<0.05). Nail brightness in the KD treatment group was improved by 33.1% and 35.7%, after 45 and 90 days; while the improvement in the BE treatment group was 16.1% and 38.7%, after 45 and 90 days. Both KD and BE nail brightness variation was statistically significant when compared to the placebo group (p<0.05). A statistically significant increase of nail growth rate was observed both in the KD and BE treatment groups (Figure 5). In the KD treatment group the nail growth rate was 0.07±0.01 mm/14 days and 0.14±0.01 mm/14 days, after 45 and 90 days, respectively (p<0.001); while in the BE treatment group nail growth rate was 0.04±0.00 mm/14 days and 0.07±0.01 mm/14 days, after 45 and 90 days, respectively (p<0.001). The nail growth rate was not statistically significant in the placebo group (p<0.05). Both KD and BE nail growth rate was statistically significant when compared to the placebo group (p<0.05). Moreover, KD group nail growth rate was statistically higher than those of BE group (p<0.05).
| Table 4. Hair and Nail Brightness |
|
n
|
Mean±SE
|
Min÷Max
|
|
KD
|
BE
|
PL
|
KD
|
BE
|
PL
|
| Hair brightness (au) |
| Day 0 |
20
|
3.36±0.41a
|
3.31±0.38a
|
3.33±0.35a
|
0.64÷6.57
|
1.45÷8.17
|
1.04÷6.71
|
| Day 45 |
20
|
3.98±0.45b
|
3.74±0.42a
|
3.28±0.36a
|
1.16÷7.44
|
1.36÷8.98
|
1.08÷6.33
|
| Day 90 |
20
|
4.99±0.53c
|
4.68±0.55b
|
3.53±0.39a
|
1.20÷8.94
|
1.91÷10.7
|
1.17÷7.63
|
| D45-D0 |
20
|
+23.8%†
|
+13.7%†
|
-1.90%
|
-12.2%÷81.3%
|
-8.1%÷47.7%
|
-24.6%÷18.3%
|
| D90-D0 |
20
|
+57.0%†
|
+41.9%†
|
6.40%
|
-6.5%÷106.3%
|
-1.1%÷88.7%
|
-22.0%÷36.5%
|
| Nail brightness (au) |
| Day 0 |
20
|
7.32±0.72a
|
7.41±0.50a
|
7.28±0.51a
|
2.17÷12.47
|
3.32÷10.84
|
4.82÷12.66
|
| Day 45 |
20
|
9.57±0.93b
|
8.63±0.70b
|
7.76±0.59a
|
3.10÷17.10
|
4.02÷16.61
|
5.00÷13.45
|
| Day 90 |
20
|
9.57±0.86b
|
10.07±0.65c
|
8.45±0.69b
|
3.44÷15.15
|
4.51÷16.05
|
5.11÷14.85
|
| D45-D0 |
20
|
+33.1%†
|
+16.1%†
|
6.70%
|
1.0%÷83.9%
|
2.1%÷84.0%
|
-16.1%÷33.9%
|
| D90-D0 |
20
|
+35.7%†
|
+38.7%†
|
17.20%
|
9.8%÷70.5%
|
5.9%÷88.1%
|
-24.1%÷66.1%
|
| Significantly Different from D0: a<b<c, p<0.05. RM-ANOVA followed by Tukey-Kramer post-test. †Significantly different (p<0.05) from Placebo. RM-ANOVA followed by tests for two-factor interactions. |
Figure 5. Nail Growth Rate
|
n
|
Mean±SE
|
Min÷Max
|
|
KD
|
BE
|
PL
|
KD
|
BE
|
PL
|
| Growth rate (mm/14 days) |
| Day 0 |
20
|
1.24±0.07a
|
1.28±0.06a
|
1.26±0.06a
|
0.68÷1.99
|
0.72÷1.82
|
0.61÷1.60
|
| Day 45 |
20
|
1.31±0.07b
|
1.32±0.06b
|
1.28±0.05a
|
0.75÷2.08
|
0.75÷1.90
|
0.78÷1.65
|
| Day 90 |
20
|
1.38±0.08c
|
1.35±0.06c
|
1.28±0.06a
|
0.78÷2.14
|
0.79÷1.92
|
0.69÷1.68
|
| D45-D0 |
20
|
+0.07†
|
+0.04†
|
0.02
|
0.01÷0.17
|
0.01÷0.08
|
-0.04÷0.17
|
| D90-D0 |
20
|
+0.14†∫
|
+0.07†
|
0.01
|
0.04÷0.27
|
0.02÷0.14
|
-0.03÷0.08
|
| Significantly different from D0: a<b<c, p<0.05. RM-ANOVA followed by Tukey-Kramer post-test. †Significantly different (p<0.05) from Placebo. ∫Significantly different (p<0.05) from Benchmark. RM-ANOVA followed by tests for two-factor interactions. Data are Mean±SE. |
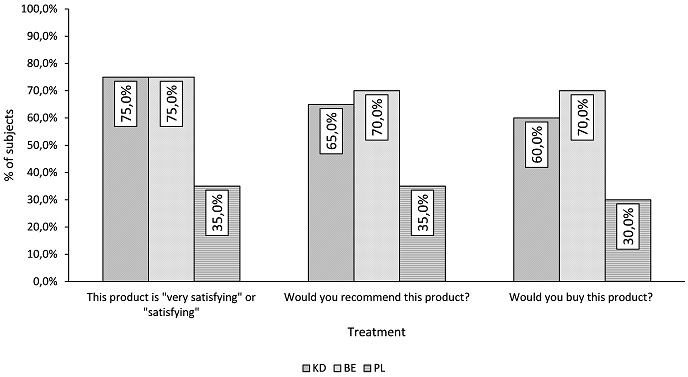
The complete results of the self-assessment questionnaire are reported in Figure 6. Both KD and BE were perceived more effective than PL. Subjects’ answers after 90 days of product use are very positive with 75% global satisfaction for both KD and BE group for hair and nails. On the other hand, placebo effect is only 35%. This underlines that all improvement of objectives criteria is enough visible to be perceived by women of KD and BE groups.
Figure 6. Self Assessment Questionnaire

DISCUSSION AND CONCLUSION
Hair and nails are two specialized keratinous skin appendages growing at fixed rate and with complete replacement over time. The growth of both hair and nails relies on the metabolic activity of the hair follicle and the nail matrix, respectively. It is then easy to understand why, virtually, every nutritional deficiency can affect the growth of the nail in some manner. The connection between nutrition and skin (and its appendages) conditions is a common interesting research field for both scientists and humans from ancient times to nowadays. It is nowadays clear that the nutrients of diet have a direct impact on the structure and growth of both hair and nails. To confirm that, in recent years the use of food supplements has increased both in Europe and in the USA with many physicians recommending them.25,26
In our study, we demonstrated the role of a food supplement containing a natural extensively hydrolyzed keratin in improving both hair and nails conditions after a 3 month intake period.
An improvement of hair conditions was seen after 90 days of treatment. This was demonstrated by the decrease of hair shedding and telogen hair. The active group showed a significant decrease of telogen hair after 45 and 90 days treatment. The percentage of hair in the telogen phase was decreased, resulting in a decrease of the number of hair loss on pull testing. Interestingly the diagnosis of aTE by pull testing was negative after 45 days treatment. Also, the hair fiber showed a positive improvement, demonstrated by the increase of its ability to reflect the light (radiance). This phenomenon relies on a healthy hair cuticle structure.
Nails also improved their conditions after 45 and 90 days of treatment. After 90 days product use the 60% of the subjects showed an improvement of nails condition. This was also demonstrated by the increase of the nail growth rate by 0.14 mm/14 days.
The active product was also scored positively by most of the subjects participating in the study. The subjects at the end of the study perceived their hair and nails conditions as improved. The efficacy of Kera-Diet® was comparable to that of the benchmark product.
The putative mechanism of action of the Kera-Diet® in improving hair and nails condition can be attributed to its association with traced elements and specific vitamins and also to the bioavailability of amino acids from Kera-Diet® which is compounded of more than 92% of free amino acids, of which the profile is close to the human keratin.
No adverse effects were neither reported by the subjects participating in the study nor by the investigator.
Therefore, and more generally, this study demonstrates that Kera-Diet® associated with traced elements and specific vitamins at the right dosage can enhance hair and nails condition, even though human nutrition is more and more balanced. The study, furthermore, demonstrates the role of nutrients in both aTE and BNS.
ACKNOWLEDGMENT
The authors would like to thank all the Complife Italia staff who contributed to and recruited subjects for this study for their professionalism and support during the study development.
FUNDING
This study was funded by BCF Life Sciences. BCF Life Sciences was involved in the design of the study protocol and provided the test products samples. Employees of the sponsor were not involved in data analysis. The manuscript was prepared by Dr. Vincenzo Nobile. BCF Life Sciences employees were permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the corresponding author. Dr. Vincenzo Nobile is the guarantor for this article and takes responsibility for the integrity of the work as a whole.
CONFLICTS OF INTEREST
The author has not received any funding or benefits from industry or elsewhere to conduct this study. Joël Duperray and Renaud Sergheraert work for BCF Life Sciences.













