INTRODUCTION
Injured athletes have a strong desire for rapid recovery from their injuries and therefore, multidisciplinary treatments are necessary to improve the healing process and to achieve an earlier return to training and competition. The goal is to establish safe procedures with low risk.
Soft tissue injuries in the acute phase are presented with localized edema and swelling, which is produced by plasma leakage from damaged blood vessels. Edema increases tissue pressure, decreases local perfusion, impairs micro-circulation, and causes hypoxia in the injured tissue.1 Ankle sprains are frequent soft tissue injuries that occur during sports activities.
The effects of HBO2 therapy on soft tissue injuries during sports activities, including sprains,2 ligament injuries,3,4,5 contusions, and muscle strains,6,7,8,9,10 have been widely reported.
HBO2 has been reported to be an effective therapy for edema reduction, which should improve local perfusion and improving oxygenation of the injured tissues.1,11 However, only Borromeo et al2 have reported clinical research data providing quantitative analysis of HBO2 therapy on ankle sprains. Their randomized control trial reported that joint function scores were more favorable in the HBO2 group, though no significant differences were observed in ankle volume or the subjective evaluation of pain. However, detailed descriptions of the effects of HBO2 therapy was missing, especially on the short-term effects of HBO2 therapy in the acute phase.
The objectives of this study were to investigate the short-term effects of HBO2 therapy on edema and to provide subjective evaluations of athletes with ankle sprains in the acute phase.
MATERIALS AND METHODS
Subjects
We performed a before-after analysis of patients who visited our clinic between the years of 2007 to 2015 with an acute ankle sprain sustained during sports activities within 7 days after the injury. The protocol for this study was approved by the Institutional Review Board of Tokyo Medical and Dental University, and informed consent was obtained from all subjects prior to participation. Forty-four patients (39 male, 5 female) were included in this study. Five of the subjects had a history of ankle sprain on the same side. The mean age of the patients was 23.2±3.8 years (range; 13-36 years).
The types of sports activities at the time of injury were as follows: Rugby (n=23), soccer (n=6), American football (n=4), baseball (n=3), and others (n=8). A diagnosis was made by the history of ankle sprains in sports activities and an assessment performed by a trained examiner (KY). The type of injury was classified as an inward twist (n=37), an outward twist (n=3), plantar flexion stress (n=3), or dorsal flexion stress (n=1) and by whether it affected the right (n=23) or left ankle (n=21). The severity of injury was classified into one of three grades; I: Stretched ligaments (not torn), with a stable joint and a negative anterior drawer test, II: A partially torn ligament with a lax joint and a partially positive anterior drawer test, III: Complete ligament rupture with an unstable joint and a positive anterior drawer test.13 In this study, the distribution of the severity of the injury was as follows: Grade 1 (n=6), grade 2 (n=35), and grade 3 (n=3). The mean number of days from injury to the first admission of HBO2 treatment was 2.1±1.2 days (0-5 days).
HBO2 Therapy Protocol and Treatment
The HBO2 chamber in our university hospital was used; the maximum capacity of our multi-place HBO2 chamber is 16 persons (NHC-412-A, Nakamura Tekko-Sho Corp., Tokyo, Japan). The HBO2 therapy protocol included pressure up to 2.5 ATA (253.3 kPa) with treatment time of 60 minutes for inhalation of pure oxygen according to the HBO2 therapy protocol for compartment syndrome (Figure 1).14 The HBO2 therapy was performed within 7 days after injury, for a maximum of 5 times within a 7-day period in all cases. In total, 110 sessions of HBO2 therapy were performed in the 44 patients (1 session (n=12), 2 sessions (n=11), 3 sessions (n=10), 4 sessions (n=9), and 5 sessions (n=2)). Other conventional treatments such as RICE (rest, ice, compression, and elevation) in the acute phase were performed concomitantly during HBO2 therapy. RICE procedures were performed during the first week of the acute phase, range of motion exercises were applied beginning one week after injury, strength and flexibility rehabilitation was applied beginning one or two weeks after injury depending on pain and edema. The patients gradually returned to activities without any recurrence of injury.
Figure 1. HBO2 Treatment Table
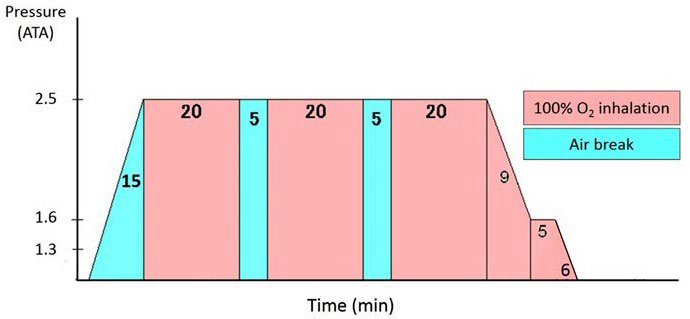
Evaluation Procedures
Foot and ankle volume measurements were used to quantitatively evaluate ankle swelling; subjective evaluations using a VAS were also performed. In order to evaluate the short-term effects of HBO2 therapy, all evaluations were performed immediately before and after the 105-minute HBO2 therapy session.
To measure foot and ankle volume, the overflow water volume was measured using a water-filled volumetric edema gauge of our own making (Figure 2). This device measured 7.5 cm wide×12.5 cm long×33 cm high, and the bottom of the outflow cylinder was placed at the height of 16.5 cm from the floor so that foot and ankle volume up to 16.5 cm from the plantar face was measured. This device improves measurement accuracy compared to existing devices by reducing the water surface area. The patients were instructed to stand-bearing the same weight on both legs. The weight of the overflow water was measured and converted to volume as 1g/cm3 specific gravity.
Figure 2. Measurement of Foot and Ankle. Measurement of Foot and Ankle by Water-Filled Volumetric Edema Gauge.
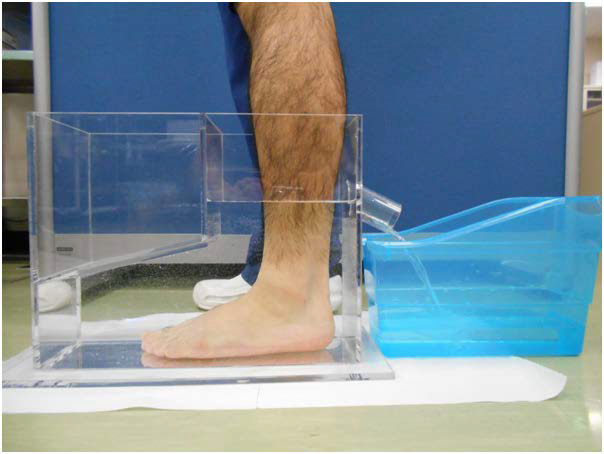
To evaluate the repeatability of the foot and ankle volume measurement, 5 volunteers (4 males, 1 female, mean age of 36.0±7.6 years; range 29-48 years old) were used. The volume measurements of the bilateral feet and ankles, a total of 10 feet and ankles, were performed 5 times each, and the Intraclass Correlation Coefficients (ICC1,5) was evaluated. The ICC1,5 was 0.997 (95% CI, 0.993-0.999).
Eighty-five measurements of the VAS out of 110 sessions of HBO2 therapy were fully recorded and were used for analysis in this study. The VAS evaluation included questions about pain at rest, pain while walking and a subjective evaluation of edema. The VAS consisted of a maximum of 100 points at full mark, where 100 points indicates the worst condition and 0 points indicates no complaint.
To assess the HBO2 therapy efficacy in detail, the foot and ankle volume and VAS measurements were evaluated taking into consideration the number of HBO2 therapy sessions and the amount of time between from the time of injury to when therapy was started.
Statistical Analysis
The data are presented as the mean±standard error of the mean (SEM). Differences were analyzed using the Paired t-test, and significance was accepted if the p-value was less than 0.05. Kruskal-Wallis one-way analysis of variance (ANOVA) tests were utilized to detect differences of foot and ankle volume in the grade of injury, the number of HBO2 sessions and duration from injury, and differences of the VAS score in the number of HBO2 sessions and duration from injury. Where appropriate, post hoc Bonferroni multiple comparison procedures were used. All data were analyzed using SPSS version 19.0 (SPSS Inc., 233 Wacker Dr, 11th Fl, Chicago, IL 60606, USA).
RESULTS
Foot and Ankle Volume
The foot and ankle volume in all sessions (n=110) were 1569.1±219.0 cm3 just before and 1557.8±218.1 cm3 just after HBO2 therapy, with a reduction of 11.3±20.8 cm3 (p<0.001) during the 105-minute HBO2 therapy. The foot and ankle volumes before the first session of HBO2 therapy and after the final session of HBO2 therapy (n=44) were 1598.1±216.8 cm3 and 1566.4±217.0 cm3, respectively, with a reduction of 31.7 cm3 (p<0.05). The foot and ankle volume before and after a HBO2 therapy session depending on the severity of the injury are presented in Table 1, and there were no statistically significant differences of reduction of the foot and ankle volume in the severity of the injury.
| Table 1. Foot and Ankle Volume Depending on Severity of the Injury. |
|
The grade of injury (n)
|
The number of HBO2 sessions |
Pre-HBO2 |
Post-HBO2 (cm3) |
reduction (cm3) § |
| Grade 1 (9) |
1.5±0.8 |
1554.9±142.3 |
1552.2±137.9 |
2.6±13.3
|
|
Grade 2 (91)
|
2.6±1.2 |
1564.2±224.9 |
1552.6±223.5* |
11.5±21.7 |
| Grade 3 (10) |
3.3±1.2 |
1626.3±230.2 |
1609.8±236.9* |
16.5±16.7
|
*: Statistical differences between pre-HBO2 and post-HBO2 performed with Paired t-test (p<0.05).
§: No statistically significant differences in the severity of injury performed with Kruskal-Wallis one-way ANOVA. |
The foot and ankle volume and reduction of volume before and after a HBO2 therapy session depending on the number of HBO2 therapy sessions are presented in Table 2. The foot and ankle volume and reduction of volume before and after a HBO2 therapy session depending on the day after injury are presented in Table 3.
| Table 2. Foot and Ankle Volume Depending on HBO2 Therapy Sessions. |
|
The number of HBO2 sessions (n)
|
Pre-HBO2 |
Post-HBO2 (cm3) |
Reduction (cm3) §
|
|
1 (44)
|
1598.1±216.8 |
1584.0±214.8* |
14.1±23.1 |
| 2 (32) |
1572.3±216.0 |
1562.6±214.4* |
9.8±19.5
|
|
3 (21)
|
1553.1±196.4 |
1542.3±202.8* |
10.8±22.3 |
| 4 (11) |
1516.0 ±248.9 |
1510.3±249.3* |
5.7±9.6
|
*: Statistical differences between pre-HBO2 and post-HBO2 performed with Paired t-test (p<0.05).
§: No statistically significant differences in the severity of injury performed with Kruskal-Wallis one-way ANOVA. |
| Table 3. Foot and Ankle Volume Depending on Duration from Injury. |
|
Duration from injury day (n)
|
Pre-HBO2 |
Post-HBO2 (cm3) R |
Reduction (cm3) § |
| 1 (11) |
1541.5±205.0 |
1521.6±206.1* |
19.9±30.2
|
|
2 (23)
|
1638.5±213.5 |
1628.4±214.9* |
10.2±22.1 |
| 3 (23) |
1598.6±219.2 |
1591.2±213.7* |
7.4±16.1
|
|
4 (20)
|
1508.2±230.2 |
1494.8±227.9* |
13.4±21.9 |
| 5 (16) |
1524.7±221.6 |
1518.1±225.3 |
6.6±15.8
|
|
6 (11)
|
1552.3±235.4 |
1540.7±229.0 |
11.6±24.9
|
| *: Statistical differences between pre-HBO2 and post-HBO2 performed with paired t-test (p<0.05).
§: No statistically significant differences in the duration from injury performed with Kruskal-Wallis one-way ANOVA. |
Visual Analog Scale (VAS)
VAS scores were 19.5±20.1 points and 17.2±19.9 for pain at rest (p<0.05), 41.5±27.3 and 30.9±24.4 for pain while walking (p<0.05), and 44.2±23.7 and 37.1±22.5 for subjective evaluation of edema (p<0.05), before and after HBO2 therapy (Table 4).
|
Table 4. VAS Scores. (Point)
|
| Pain at rest |
Pain while walking |
Subjective evaluation of edema
|
|
Pre-HBO2
|
Post-HBO2 (cm3) |
Pre-HBO2 |
Post-HBO2 (cm3) |
Pre-HBO2 |
Post-HBO2 (cm3) |
| 19.3±20.1 |
17.2±19.9* |
41.5±27.3 |
30.9±24.4* |
44.2±23.7 |
37.1±22.5*
|
| *: Statistical differences between pre-HBO2 and post-HBO2 performed with paired t-test (p<0.05). |
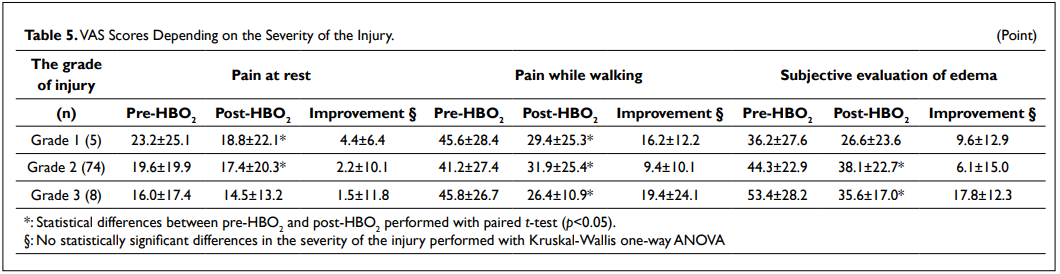
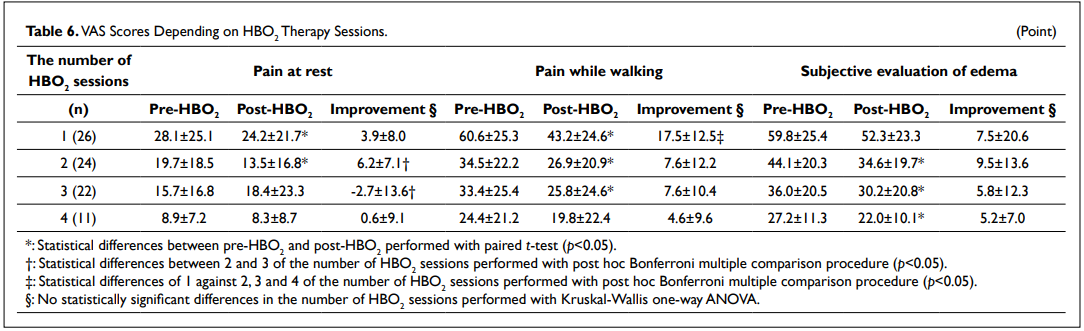
When considering the severity of the injury, there were no statistically significant differences of improvement between groups (Table 5). When considering the number of therapy sessions, the VAS scores for pain while walking were 60.6±25.3 points and 43.2±24.6 (p<0.05) for the first HBO2 therapy session (n=26) and 33.4±25.4 points and 25.8±24.6 (p<0.05) for the third HBO2 therapy session (n=22) before and after an HBO2 therapy session, respectively (Table 6). There were statistically significant differences of 1 against 2, 3 and 4 of the number of HBO2 sessions regarding to the VAS scores for pain while walking (p<0.05).
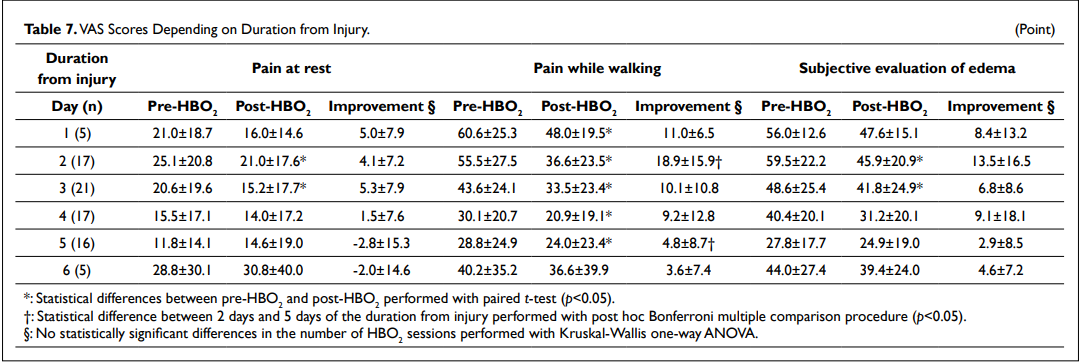
With respect to the time after injury when treatment began, the VAS scores for pain while walking were 55.5±27.5 points and 36.6±23.5 (n=17) when started 2 days post injury (p<0.05) and 28.8±24.9 points and 24.0±23.4 (n=16) 5 days post injury (p<0.05) just before and after HBO2 therapy, respectively (Table 7). There was a statistically significant difference between 2 days and 5 days of the duration from injury regarding to the VAS scores for pain while walking (p<0.05).
DISCUSSION
In the present study, we administrated HBO2 therapy to athletes with acute ankle sprains and quantitatively evaluated the shortterm effects of HBO2 therapy in the acute phase, though the RICE treatment is also effective in the acute phase, and HBO2 therapy and the RICE treatment are confounding factors correlating to the outcomes.
The results of this pilot study revealed significant edema reduction of 11.3 cm3 and significant improvement in the VAS scores for pain at rest, pain while walking and subjective evaluation of edema after HBO2 therapy.
We also evaluated the effectiveness of HBO2 therapy as it relates to the severity of the injury, the number of HBO2 therapy sessions and the duration of time from injury to the start of treatment. HBO2 therapy was most effective at the first application with an average reduction of 14 cm3 in foot and ankle volume, and with an improvement of 17.5±12.5 points in the VAS scores for pain while walking, which was statistically significant differences against the second, third and fourth application. Edema reduction of more than 10 cm3 was observed within 3 sessions, and the significant reductions in the VAS scores were observed within 4 sessions. Edema reduction of more than 10 cm3 was observed within 4 days from injury and significant reductions in the VAS scores were observed when treatment started within 5 days from injury. Too long HBO2 therapy per session should be avoided due to the possibility of the manifestation of oxygen toxicity, even though the possibility is very low.15
Basic and clinical studies of HBO2 therapy for soft tissue injuries, mainly for sprains,2 ligament injuries3-5 and muscle injuries6-10 that occurred during sports activities, have been reported. Regarding to the effects of HBO2 therapy on edema in the acute phase, Skyhar et al1 examined the effects of HBO2 therapy using a dog hemorrhagic model for acute compartment syndrome and reported that it significantly reduced edema and occurrence of muscle necrotic tissue. They speculated that those findings might be the result of hyper-oxygenation and vasoconstriction. Hyper-oxygenation increases the amount of oxygen in the peripheral tissue without hemoglobin involvement, and vasoconstriction reduces blood flow by 20% while outflow is maintained.12 This resulted in an increase of resorption of the extra-vascular solution, and the net effect was edema reduction. Strauss et al11 induced compartment syndrome in the hind limbs of 12 dogs and revealed a reduction of muscle necrosis and edema in the experimental compartment. According to these past reports, it is expected that HBO2 therapy will accelerate the reduction of edema by oxygenation of the localized hypoxic environment and that HBO2 therapy can result in an earlier withdrawal from the vicious circle of localized hypoxia and edema in the acute phase.
In patients with ankle sprains, ankle edema increases the sensation of pain and restricts the ankle range of motion leading to delayed rehabilitation.16 Multidisciplinary treatments including RICE are required for rapid reduction of ankle edema, especially for high-performance athletes who need accelerated healing in order to return to competition as soon as possible.
In a previous clinical study of HBO2 therapy for acute ankle sprains, Borromeo et al2 reported a randomized control trial for patients with acute ankle sprain, and HBO2 treated group receiving oxygen at 2.0 ATA (202.7 kPa) compared to a control group who received air at 1.1 ATA (111.5 kPa). They reported that joint function scores were more favorable in the HBO2 group, although no significant differences were observed for ankle volume, subjective evaluation of pain, or range of ankle movement. As we applied an HBO2 therapy protocol of 2.5 ATA (253.3 kPa), a slightly higher pressure than that used by Borromeo2 we expected to see significant effects of HBO2 therapy.
We recognize that a fundamental limitation of this study is the lack of a control group and therefore we could not definitively conclude the superiority of HBO2 treatment over the conventional treatment including RICE therapy without HBO2. Thus, we reported this study as a pilot study. The second limitation of this study was the absence of evaluation of the long-term effects of HBO2 therapy on ankle sprains. Time to return to competition was not measured and was not compared to a control group or past reports. Additionally, the VAS evaluation is highly subjective and the placebo effects cannot be discounted; this limitation is also related to the lack of a control group. Lastly, a potential limitation of this study is an incomplete collection of VAS measurements from all subjects.
Future studies should determine whether HBO2 therapy can accelerate healing processes and reduce the period for return to competition.
CONCLUSION
This pilot and observational study in athletes with ankle sprains in the acute phase found that a reduction of foot and ankle volume was measured with 11.3±20.8 cm3 during the 105-minute HBO2 therapy, and the VAS sores were measured with 19.5±20.1 points and 17.2±19.9 for pain at rest (p<0.05), 41.5±27.3 and 30.9±24.4 for pain while walking (p<0.05), and 44.2± 23.7 and 37.1±22.5 for subjective evaluation of edema (p<0.05) before and after HBO2 therapy.
ACKNOWLEDGEMENTS
We specially thank Dr. Takashi Hirai, Dr. Yasushi Kojima, Dr. Takashi Taniyama, and all the medical engineers of Hyperbaric Medical Center in Tokyo Medical and Dental University for supporting this study. This work was partially funded by the Yamaha Foundation for Sports.
CONFLICTS OF INTEREST
The authors have declared that no conflict of interest exists with this submission.
ETHICAL CONSIDERATIONS
This study was approved by the Institutional Review Board of Tokyo Medical and Dental University in 2005, and all patients signed an informed consent form before participation. This study was undertaken in full accordance with the ethical standards in the Helsinki Declaration.










