INTRODUCTION
Trigeminal neuralgia (TN) is one of the most painful diseases known to man. The third edition of the International Classification of Headache Disorders (ICHD-3) describes TN as “a disorder characterized by recurrent unilateral brief electric shock-like pains, abrupt in onset and termination, limited to the distribution of one or more divisions of the trigeminal nerve and triggered by innocuous stimuli”.1
The incidence of TN is 4-29 per 100,000 persons per year worldwide.2,3,4,5 TN prevalence in the population is 0.07% compared to the prevalence of facial pain that is approximately 2%.4,5
The most recognized theory regarding the pathophysiology of TN is a demyelination of the sensory fibers of the trigeminal nerve. Walter Dandy first made the observation that the nerve was compressed by vascular structures.6 A century later the suspicion was confirmed: in most cases of TN a blood vessel compresses the nerve root7 triggering the injury to the trigeminal axons. The arteries most frequently involved are the superior cerebellar artery (75%) and to a lesser extent, the inferior cerebellar artery (10%).8
The international headache society (IHS) classifies TN into two categories: classic TN and symptomatic (secondary) TN.9 Classic TN develops without an identifiable cause, other than a vascular compression of the trigeminal nerve. Symptomatic TN includes patients in whom an identifiable cause can be found, such as tumors, trauma or arteriovenous malformation.9,10,11,12 Some authors suggest an additional differentiation of idiopathic TN from classic TN, when a vascular compression of the trigeminal nerve is not found and an etiology cannot be identified.13,14
Therapies for TN and neuropathy have often been pharmacologic or surgical.15,16 Antiepileptic drugs are the first line of treatment in TN. According to current evidence, carbamazepine (CBZ) is the first choice of treatment, established as effective (level A) and the only medication approved by the Food and Drug Administration (FDA) for TN. Oxcarbazepine is considered probably effective (level B) while baclofen and lamotrigine are possibly effective (level C).17,18,19 Pharmacologic therapies are not successful in some cases and often cause side effects.20
When pharmacologic therapies fail and more aggressive treatment is pursued, surgery is often considered.21 There are several surgical therapies available and the type of technique depends on the institution and the surgeon who performs them as well as on the characteristics of each patient.22 Microvascular decompression is considered the gold standard and has the least pain recurrence, however it is an invasive procedure, with associated morbidity and mortality, in addition to relapses. Percutaneous rhizotomy, percutaneous balloon compression, and stereotactic radiosurgery are other options that can be considered for poor operative candidates.23
Because of the limitations associated with conventional treatment, the use of complementary and alternative interventions has increased steadily, as is the case with transcutaneous electrical nerve stimulation (TENS)24 procedures and low level laser therapy.25 Both of these methods have been shown to increase messenger ribonucleic acid (mRNA) precursors, inducing an analgesic effect.25,26
Transcutaneous electrical nerve stimulation (TENS) is a noninvasive, non pharmacological, and low-cost analgesic resource, defined by the American Physical Therapy Association (APTA) as the application of electrical stimulation to the skin for pain control.27 Since 1970s TENS has been used in acute and chronic pain, managing to reduce the intensity of pain, as well as the consumption of analgesics.28 TENS works through activation of m and d opioid receptors in the spinal cord, bulb and probably the periaqueductal gray matter.29
TENS is a safe and promising treatment option in TN, with no noticeable side effects beyond contact dermatitis caused by self-adhesive electrodes, almost no interaction with other treatments, and few contraindications.30 It is a low risk, high benefit procedure and, if necessary, can be easily halted.31 Since TENS stimulates both sensory and motor fibers, its analgesic effects are both peripheral and central.32
TENS allows for regulation of frequency and intensity, which is a valuable tool for pain control as different endogenous opioids are released depending on the frequency of TENS.33 In general, a higher intensity is more effective than a lower one, however it should not be excessive since tolerance to stimulation diminishes the analgesic effect.34
The aim of this study was to determine if the treatment of refractory TN with TENS compared to CBZ could lead to lower visual analog scale (VAS) and barrow neurological institute (BNI) pain scale scores, a reduction in number and duration of pain episodes and a lower daily analgesic intake.
PATIENTS AND METHODS
This randomized, double blinded placebo-controlled clinical trial was approved by the Ethics Committee of the “Hospital General de Zona 1” of Colima, Mexico and was carried out in accordance with the principles of the Helsinki declaration.35
From January 2000 to December 2005 patients who presented to the Pain Management Department of the “Hospital General de Zona 1” with refractory TN previously treated by their primary care physician, were screened for inclusion in the study. Patients were considered “refractory” to pharmacological treatment when adequate doses of CBZ in addition to oxcarbazepine, gabapentin or pregabalin administered for a period of 3 to 6 months, failed to achieve pain control.36
A routine head Magnetic resonance imaging (MRI), focusing on the trigeminal nerve was taken for each patient, including a 3 dimensional (3D) perpendicular trigeminal rendering to detect neurovascular compression; also transversal sagittal T1 images and T2 weighed coronal images were obtained, in order to discern between vascular and nerve structures.
Twenty-five patients who met the criteria were identified; one was excluded for having a posterior fossa vascular malformation and four others chose not to participate. Twenty patients diagnosed with classic TN of the 2nd and 3rd unilateral division in accordance to the IHS guidelines,1 were enrolled in the study. All patients signed consent forms and agreed to discontinue any analgesic or antiepileptic medication prior to enrollment. Participants were informed of possible side effects experienced with TENS such as skin irritation, as well as possible side effects associated with CBZ such as dizziness and nausea. They were also informed that they could withdraw from the study at any point with no explanation required.
Inclusion Criteria
Patients were enrolled based on the following inclusion criteria: 1) men and women older than 18 years of age; 2) diagnosis of TN with symptoms presenting over 6 months or more; 3) reported moderate to severe pain, with Visual Analogue Scale (VAS) score≥6 out of 10; 4) previously unsuccessful treatment; 5) no standing contraindications for TENS according to the Chartered Society for Physiotherapy (CSP)(UK) guidelines for safe use of electrophysical agents.37 6) ability to understand and sign an informed consent form.
Exclusion Criteria
Patients were excluded based on the following criteria: 1) secondary TN; 2) serious psychiatric or psychological condition or a Folstein Mini-Mental (FMM) test below 24 points; 3) acute pain of a different origin such as low back pain or fibromyalgia; 4) previous treatment of acute or chronic pain conditions with TENS; 5) tumors, vascular intracranial lesions, multiple sclerosis or herpes zoster; 6) patients with pacemakers; 7) an epilepsy history; 8) concurring pregnancy.
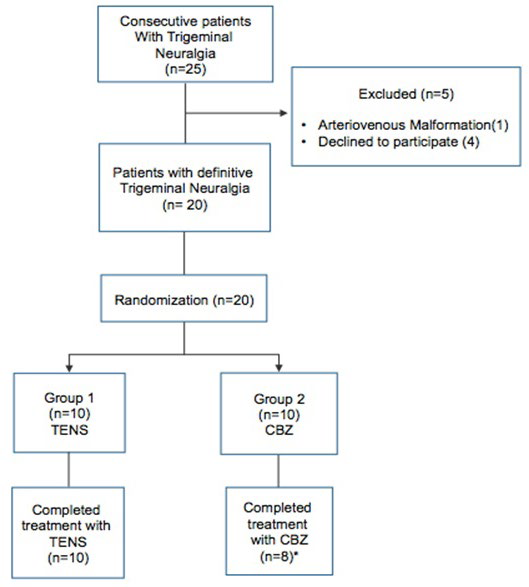
Once diagnosed and enrolled, patients were randomized into one of the two treatment groups: 10 patients were assigned to Group 1 (TENS) and 10 patients to Group 2 (CBZ). Randomization was carried out by means of a random numbers table prepared independently by a computer (Figure 1).
Figure 1. Flow Diagram of Participants Through the Study. TENS-Transcutaneous Electrical Nerve Stimulation; CBZ-Carbamazepine.
*Two Patients in the CBZ Group were Lost to Follow-up
Group 1:
Patients in the TENS group remained comfortably seated during the procedure, which was always performed by the same experienced researcher. After cleansing of the face, two adhesive electrodes were placed on the skin over the painful area and plugged to the stimulator through 4 electrodes. The first electrode was placed on V1, the second on V2 inward to the midline of the nasolabial fold, the third was placed in the preauricular region and the fourth was placed on V3. Each session lasted for 60 minutes. When correctly applied, TENS therapy should be “strong but comfortable”.34,38 In order to achieve this, participants were instructed to say “Detect” when they first perceived the stimuli and “Pain” when they found the stimuli to be painful. Before the experiment, participants were informed that visible muscle contractions may occur during TENS administration.39,40 The stimulus intensity was increased according to patient tolerance as to not cause discomfort and only slight paresthesia and muscle contraction at the area of facial pain, with a frequency between 50 and 100 Hz, a pulsating rate of 100-150 pps, and pulse duration of 50 to 200 μs. To familiarize patients with the stimuli, a low frequency was started and incremented up to 100 Hz. This group also received 3 pills a day of oral placebo, every 8 hours.
Group II:
This group received oral CBZ. The starting dose was 100 mg daily, which was then increased to 100 mg twice daily. The dose was increased by 100 mg every other day until achieving a maintenance dose of 600 mg daily (given in 3 divided doses). Although the recommended highest dose is 1200 mg/d,41 the appearance of uncomfortable side effects in our patients prevented further upward titration. Patients also received inactive TENS, which was to act as placebo. TENS electrodes were placed and connected to the stimuli generator, however the device remained inactive during the 60 minutes of the therapy. We used several techniques to prevent unblinding such as the inclusion of patients who were unfamiliar with TENS therapy and the use of a device that displayed an activating light without delivering any current. All patients were examined on separate days, so no communication between patients ever took place. Patients remained blind to the treatment until the end of the statistical analysis.
To prevent any unblinding within the research team, the physician who administered active TENS or “placebo” TENS was the only one in the know as to what kind of treatment each patient was receiving. The researcher informed patients of the first group that they should feel painless electrical stimulation, whereas he informed patients belonging to second group that they were not expected to experience electrical stimulation of any kind. The same researcher performed randomization and treatment. There was a treating physician and a third researcher analyzing the results, to maintain study blindness. During the second month of the study, daily TENS therapy was switched to an alternate day treatment, while maintaining daily pill therapy, either CBZ or placebo. In the third month TENS therapy was switched to twice a week, while continuing daily pill therapy (CBZ or placebo, accordingly). All patients were free to take 500 mg acetaminophen tablets if they had moderate to severe pain. The treatment period had a three-month duration for both groups, with a pain response evaluation done at three intervals: pre-intervention (basal values), mid-intervention (1 month), and post intervention (3 months). Once treatment period was over, follow-up of the patients was performed as an outpatient visit, once a month for the first 6 months and thereafter once every 6 months during the 5 years follow-up. The time points that we considered for the statistical analysis were 2 months, 3 months, 1 year, 3 years and 5 years after initiating treatment.
The TENS unit we used ((Model 120Z; Medtronic, Inc., Minneapolis, MN, USA) provided a symmetric biphasic pulse waveform with pulse duration of 100 ms. Frequency was modulated from 1 to 250 Hz.
Outcomes
1. The severity of pain was evaluated using a Visual Analogue Scale (VAS) score (from 0 indicating no pain, to 10 indicating the worst pain).
2. Clinical results were classified according to the Barrow Neurological Institute (BNI) pain scale. The BNI pain scale has 5 levels:
I. Excellent (complete disappearance of pain and no medication required);
II. Mild pain (no medication required);
III. Moderate pain (medication required for complete control),
IV. Moderate pain (medication required but incomplete control);
V. Severe or unrelieved pain.
A BNI score of I to III indicated satisfactory pain relief, while a BNI score of IV to V indicated poor pain relief, which serves as a criterion for evaluating recurrence after surgery.42
3. Duration of pain episodes measured in minutes.
4. Number of pain episodes per day.
5. Daily analgesic intake measured in number of acetaminophen 500 mg tablets.
STATISTICAL ANALYSIS
Descriptive statistics were calculated for all variables. Continuous data are presented as mean±SEM and as medians and ranges. Categorical data are presented as frequencies and percentages. Chi square tests were used to compare BNI and VAS scores. Group comparisons for continuous variables were examined using independent t-tests when data were normally distributed and using Mann-Whitney U-test when not normally distributed. Two-way ANOVA with repeated measurements was used to evaluate the effect of the intervention (TENS, CBZ) and assessment time points (2 months, 3 months, 1 year, 3 years and 5 years).
Friedman test was used for nonparametric data, and when a difference was detected between groups. The association between TENS effect on pain control and time was measured using the Kaplan-Meier method and the log-rank test. p<0.05 indicated statistical significance. Statistical analysis was performed using the Statistical Package for the Social Sciences version 16.0 (SPSS, Chicago, IL, USA).
RESULTS
Patient Characteristics and Trial Profile
A total of 20 patients with a diagnosis of TN were enrolled in the study. There was no statistically significant difference in demographic profile or in clinical baseline characteristics (Table 1). Of the 20 patients enrolled, 18 completed the study and were analyzed for efficacy of treatment. Two patients from group 2 were lost to follow-up; they refused to continue the study because they considered the intervention ineffective (Figure 1).
| Table 1. Patients’ Demographic Information |
|
TENS group (n=10)
|
CBZ group (n=10) |
p value
|
| Female/Male ratio |
3/7
|
3/7 |
1.0
|
| Age (y) |
64.9 ± 14
|
65.4 ± 13 |
0.8
|
| Duration of pain |
2.1 ± 0.73
|
1.9 ± 0.56 |
0.37
|
| VAS Baseline |
9.0 ± 1.333
|
9.40 ± 1.075 |
0.470
|
| BNI Baseline |
4.6 ± 0.69
|
4.5 ± 0.7 |
0.754
|
| Paroxysms/Day Baseline |
5.1 ± 1.2
|
4.9 ± 1.1 |
0.58
|
| Duration of Event Baseline |
1.7 ± 0.4
|
1.65 ± 0.3 |
0.7
|
| Analgesic intake per day Baseline |
5.1 ± 1.2
|
4.4 ± 1.3 |
0.87
|
| Patient’s demographic data by group. Results are reported as mean±standard deviation (SD) or actual ratio. VAS, Visual Analogue Scale; BNI, Barrow Neurological Institute. *Statistical significance is defined as p<0.05. |
VAS Pain Score
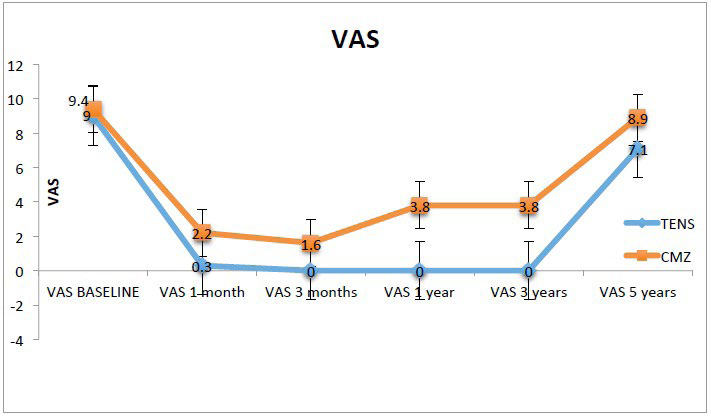
When comparing the two groups, it was found that the TENS group had a lower mean VAS score than the CBZ group for all time points. At 2 months the TENS group had a mean VAS score of 0.3, while the CBZ group had a mean VAS score of 2.2 (p=0.005). The mean VAS score at 3 months, 1 year and 3 years for the TENS group was 0, while the CBZ group had a mean VAS score or 1.6, 3.8 and 3.8 respectively. The difference between groups was statistically significant for the 3 time points (p=0.016, p<0.0001, p<0.001). At 5 years the mean VAS score for the TENS group was 7.1 while the CBZ group had a score of 8.9, no statistically significant difference was found with a p value of 0.065 (Table 2, Figure 2).
| Table 2. Visual Analogue Scale (VAS) Pain Score |
|
Time point
|
TENS group (n=10) |
CBZ group (n=10) |
p value
|
| Baseline |
9.0 ± 1.333
|
9.40 ± 1.075 |
0.470
|
| 2 months |
0.3 ± 0.949
|
2.2 ± 1.619 |
0.005*
|
| 3 months |
0
|
1.6 ± 1.8 |
0.016*
|
| 1 year |
0
|
3.8 ± 1.03 |
0.000*
|
| 3 years |
0
|
3.8 ± 1.03 |
0.000*
|
| 5 years |
7.1 ± 2.47
|
8.9 ± 1.52 |
0.065
|
| Visual Analogue Scale (VAS) pain score at baseline, 2 months, 3 months, 1 year, 3 years and 5 years. Results are reported as mean ± standard deviation (SD). *Statistical significance is defined as p<0.05. |
Figure 2. Visual Analogue Scale (VAS) Pain Score. VAS Pain Score Graph from Baseline to End of Treatment for Both Groups. Each Point Represents the Mean VAS Pain Score for that Time Point
BNI Pain Score
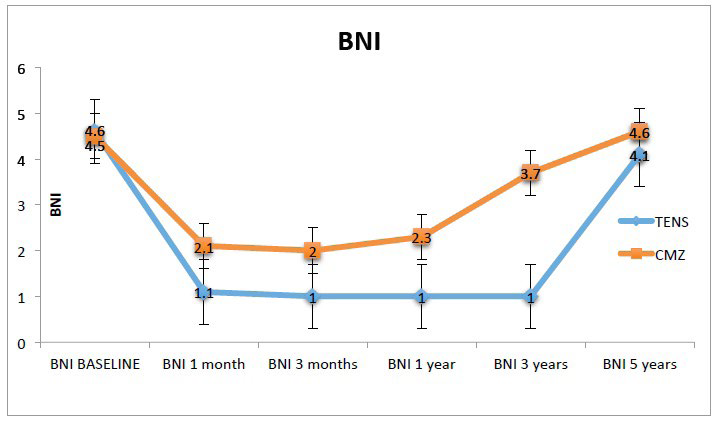
As show in Table 3, the difference between both study groups with respect to the mean BNI scale was statistically significant at 2 months, 3 months, 1 year and 3 years (p=0.007, p=0.008, p<0.001, p<0.001). Although the mean BNI scale was slightly lower in the TENS group than in the CBZ group at 5 years, this difference was statistically non significant (p=0.137). The TENS group remained pain free for up to 3 years (Table 3).
| Table 3. Barrow Neurological Institute (BNI) Pain Scale Score |
|
Time point
|
TENS group (n=10) |
CBZ group (n=10) |
p value
|
| Baseline |
4.6 ± 0.699
|
4.5 ± 0.707 |
0.754
|
| 2 months |
1.1 ± 0.316
|
2.1 ± 0.994 |
0.007*
|
| 3 months |
1± 0
|
2 ± 1.054 |
0.008*
|
| 1 year |
1 ± 0
|
2.3 ± 0.949 |
0.000*
|
| 3 years |
1 ± 0
|
3.7 ± 0.675 |
0.000*
|
| 5 years |
4.1 ± 0.876
|
4.6 ± 0.516 |
0.137
|
| Barrow Neurological Institute (BNI) pain scale score at baseline, 2 months, 3 months, 1 year, 3 years and 5 years. Results are reported as mean ± standard deviation (SD). *Statistical significance is defined as p<0.05. |
The TENS group showed an excellent response to treatment (BNI I, complete disappearance of pain and no medication required) starting at 3 months and up until 3 years after treatment while the CBZ group had a mild response to treatment (BNI II, mild pain and no medication required) at 3 months and 1 year, and experienced moderate pain (BNI III, medication required for complete control) at 3 years. At 5 years patients in both groups suffered moderate pain (BNI IV, medication required but incomplete control) (Figure 3).
Figure 3. Barrow Neurological Institute (BNI) Pain Scale Score. BNI Pain Score Graph from Baseline to End of Treatment for Both Groups. Each Point Represents the Mean BNI Pain Score for that Time Point
Duration of Pain Episode
The duration of pain episodes was significantly higher at 2 months and 3 years in the CBZ group when compared to the TENS group (p=0.001, p=0.010). At 5 years the duration of pain episodes appeared to be significantly higher in the TENS group instead (p=0.028). No statistical difference was found at 3 months or 1 year between groups (p=0.178, p=0.172).
Number of Episodes Per Day
When comparing the number of pain paroxysms per day between groups, it was found that the TENS group showed a lower number of episodes at all time points. There were no pain episodes reported in the TENS group at 3 months, 1 year and 3 years, while the CBZ group experienced a mean of 0.9, 2.3 and 3.2 events for the same time points. The difference between groups was statistically significant (p=0.019, p=0.001, p<0.001). At 5 years, patients in the TENS group had a mean number of events of 3.8 while patients in CBZ had 3.9 events, with no statistically significant difference between them (p=0.844) (Table 4).
| Table 4. Number of Pain Paroxysms per Day |
|
Time point
|
TENS group (n=10) |
CBZ group (n=10) |
p value
|
| Baseline |
5.1 ± 1.28
|
4.9 ± 1.10 |
0.583
|
| 2 months |
0.2 ± 0.632
|
1.3 ± 1.05 |
0.011*
|
| 3 months |
0
|
0.9 ± 1.10 |
0.019*
|
| 1 year |
0
|
2.3 ± 1.76 |
0.001*
|
| 3 years |
0
|
3.2 ± 0.919 |
0.00*
|
| 5 years |
3.8 ± 1.033
|
3.9 ± 1.197 |
0.844
|
| Results are reported as mean ± standard deviation (SD). *Statistical significance is defined as P < 0.05. |
Regarding analgesic intake, the TENS group showed a reduction in the number of acetaminophen tablets required for pain control, when compared to the CBZ group at 2 months, 3 months, 1 year and 3 years (p=0.000, p=0.000, p<0.0001, p<0.001 respectively). While there was no statistically significant difference between groups at 5 years (p=0.588), mean analgesic intake was increased in the TENS group (9.9) versus the CBZ group (3.1) (Table 5).
| Table 5. Analgesic Intake (Number of Tablets/Day) |
|
Time point
|
TENS group (n=10) |
CBZ group (n=10) |
p value
|
| Baseline |
5.1 ± 1.20
|
4.4 ± 1.30 |
0.87
|
| 2 months |
0
|
2 ± 1.15 |
0.000*
|
| 3 months |
0
|
1.8 ± 1.13 |
0.000*
|
| 1 year |
0
|
1.8 ± 0.019 |
0.000*
|
| 3 years |
0
|
1.8 ± 0.919 |
0.000*
|
| 5 years |
9.9 ± 0.738
|
3.1 ± 0.876 |
0.588
|
| Results are reported as mean ± standard deviation (SD). *Statistical significance is defined as p<0.05. |
After initiating treatment, patients with complete resolution of symptoms in the TENS group and in the CBZ group accounted for 60% and 50% at 1 month, 100% and 77.77% at 3 months, 100% and 62.5% at 1 year, 100% and 50% at 3 years, respectively. By the 5-year mark, 100% (n=10) of patients in the TENS group and 100% (n=8) of patients in the CBZ group had experienced pain recurrence. (Table 6)
| Table 6. Percentage of Pain-Free Patients |
|
Time point
|
TENS group (n=10) |
CBZ group (n=10) |
p value
|
| 1 month |
60%
|
50% |
0.172
|
| 3 months |
100%
|
77.77% |
<0.05*
|
| 1 year |
100%
|
62.5% |
<0.05*
|
| 3 years |
100%
|
50% |
<0.05*
|
| 5 years |
0%
|
0% |
0.588
|
| 5 years |
7.1 ± 2.47
|
8.9 ± 1.52 |
0.065
|
| Visual Analogue Scale (VAS) pain score at baseline, 2 months, 3 months, 1 year, 3 years and 5 years. Results are reported as mean ± standard deviation (SD). *Statistical significance is defined as p<0.05. |
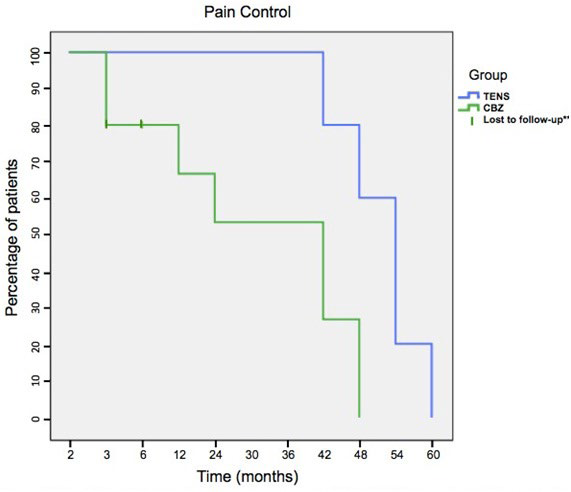
The TENS group achieved better pain control than the CBZ group for the first three years (as inferred by the inferior number of pain events, lower pain scores and lower analgesic intake) and the difference was statistically significant (p<0.05). The first patient in the TENS group to experience pain recurrence, did so after the 3 years mark while the first patient in the CBZ suffered an event by the 1st year (Figure 4).
Figure 4. Kaplan Meier Plot for Pain Control and Pain Recurrence in TENS and CBZ Groups. Patients Follow-up was for 5 Years (60 months) After End of Treatment. ** Two Patients in the CBZ Group were Lost to Follow-up
Side Effects
Six patients in the CBZ group presented nausea, vertigo and vomiting; side effects were not serious and spontaneously disappeared altogether. Patients in the TENS group experienced no side effects.
DISCUSSION
TN is a debilitating chronic pain disorder and in refractory patients, it can severely impair quality of life. CBZ remains the first-line recommended therapy, with surgery as an alternative treatment option after loss of pharmacological effect, intolerance to the medication or refractory pain. Complications following surgery include paresthesias, facial palsy, hearing loss, cerebellar injury, CSF leak, hematoma and infection. Long-term follow-up of surgical patients has shown a total cure rate of 60-88% with a relapse rate between 10-54% after 3 years. 23,43
Several studies have validated TENS as an effective treatment option for chronic and acute pain,44,45,46 however, there is a significant lack of studies that have explored the administration of TENS as a possible therapy in TN.47,48 The findings of our study suggest that TENS is an valuable therapeutic alternative for patients with refractory TN. Patients that received TENS exhibited significant improvement in pain scores and lower analgesic intake. TENS proved to be not only effective in diminishing the VAS and BIN pain scores once treatment was initiated, but also in decreasing the number of pain events. It is interesting to note that after 3 months of treatment, patients reported no pain paroxysms and they remained episode-free for the following 3 years. There is reason to believe there could be a cumulative effect of multiple doses of TENS in pain relief.49
In contrast, patients who received CBZ continued with a variable number of events and reported higher VAS and BNI scores. It was only after 3 years following TENS treatment when patients’ VAS and BNI scores, as well as the number of events, duration of events and amount of analgesic tables required for pain control, became similar between the two groups.
A TENS machine is a small and practical battery-operated device that can be purchased or borrowed from a pain and physiotherapy clinic and administered at home once the patient is taught to apply it properly.50 The simplicity of the procedure, the low long-term cost and the absence of major side effects, as well as the avoidance of surgical and anesthetic risks, suggest that TENS can be an excellent option in refractory TN.
We acknowledge our study has several limitations. The sample size of the subjects is insufficient for generalization of the results. Further studies may want to investigate the effects of TENS on refractory TN with a larger sample size.
Another limitation of the study is the use of CBZ in patients who had failed to obtain adequate control with previous administration of this drug. However, since initial management of patients was not carried out by our pain clinic, we could not be certain that appropriate doses had been employed. For this reason, and because CBZ is the only drug FDA approved for TN treatment,51 we chose to use it for the control group regardless of previous background.
Although it is possible that a higher dose of CBZ in refractory TN patients could help achieve better pain control when a lower dose has not,18 administering more than 600 mg/d was not an option for our patients because higher doses were not tolerated.
CONCLUSION
The results of our study suggest that TENS is a safe, simple, and effective treatment option for achieving pain control in refractory TN and showed excellent patient tolerability, with no notable adverse effects. TENS is a non-invasive and inexpensive technique and its potential for reducing healthcare costs and improving quality of life warrants further research. Clinical trials with a larger sample size are needed to evaluate the role TENS therapy could play in the management of TN.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









