INTRODUCTION
Gastroptosis was a frequent diagnosis in former days, however in current practice it is a rare diagnosis with uncertain etiology. Gastroptosis is diagnosed by the downward displacement of the stomach on an upper gastrointestinal (GI) study in a standing position, with the greater curve of the stomach partly projecting below the level of the iliac crests (Figure 1).1,2 The physiological position of the stomach varies between individuals, and also in one subject depending on many factors like stomach tone, the degree of fullness of the stomach, the position of the subject (supine or upright) and the strain of the abdominal muscles.3
Figure 1: Gastroptosis on an Upper-Gastrointestinal Study
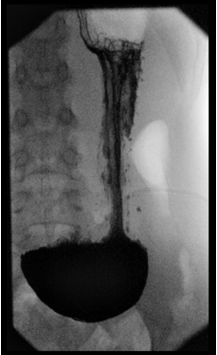
Gastroptosis may be associated with a variety of GI symptoms including epigastric pain or discomfort, early satiety and acid brash.1,2,3,4 After a meal patients with gastroptosis may suffer from nausea and discomfort.1 Gastroptosis is also associated with delayed gastric emptying.1,3 Delayed gastric emptying, altered antroduodenal motility, and impaired gastric accommodation have been proposed to explain symptoms of functional dyspepsia.5,6,7,8,9 It is not known if gastroptosis itself leads to certain symptoms or if gastroptosis and related symptoms are the result of another disease. Symptoms related to gastroptosis are non-specific and could be caused by many other conditions.
In the past, surgical treatment was preferred.10 Now-a-days only few cases have been reported in the literature.1,3 In our university hospital, we recently were confronted with some children suffering from GI symptoms who had gastroptosis on the upper GI study, which brought this condition to our attention. Only small studies are available on gastroptosis, mostly on adults, and most of them are outdated.
It is not clear if gastroptosis is still a relevant diagnosis to explain certain GI symptoms. This study aims to study the possible association of gastroptosis and GI symptoms in children.
METHODS
Population
We retrospectively investigated the upper GI tract studies of children aged 6 to 18 years in the Radboudumc Amalia Children’s Hospital between March 2010 and March 2015. Children with a history of fundoplication were excluded, because of the change in stomach position and anatomy. Fundoplication can result in decreased gastric accommodation, which may be associated with postprandial fullness and dyspeptic symptoms,9 which could bias the control group. Children with a history of or an indication for gastrostomy were also excluded.
The diagnosis of gastroptosis had to be established onan upper GI study in a standing position, therefore children who were studied only in supine position were excluded.
Data Collection
All data were extracted from electronic patient files (EPIC Hyperspace PRD 2014), including the age on the date of the upper GI study, gender, height standard deviation score (SDS), body mass index (BMI, kg/m²) and BMI SDS of within 2 months of the upper GI study. The BMI SDS represents the deviation in BMI from the mean BMI of the general population of children with the same age and gender. To be able to compare BMI between the children and the groups, BMI SDS was used to correct for gender and differences in age.
GI symptoms were assessed by investigation of the electronic patient files, particularly for a period of 6 months before the enema study was done. Possible symptoms of gastroptosis recorded in the database included epigastric pain or discomfort, acid brash or heartburn, nausea, constipation, recurrent diarrhea (>2 times a week), recurrent vomiting (>2 times a week), abdominal distension and early satiety.
Gastroptosis was diagnosed by anupper GI study either with barium or iodinated water soluble contrast, using pulsed fluoroscopy and/or radiographs, according to the insights of the performing pediatric radiologist. Images of the upper GI study were studied for each child using the image viewer of Impax (Agfa). The diagnosis of gastroptosis was set by the investigator if the major curve of the stomach was partly located below the level of the iliac crests in a standing position, measured by the drawing of ahorizontal line between the iliac crests. In a supine position, the stomach could be positioned normally, while the stomach descends when the patient stands up.11 To determine the position of the patient during the upper GI study, all images were investigated on the presence of air-fluid levels. Children with images only in supine position were excluded. The group of children with gastroptosis on the upper GI study was compared to the group of children without gastroptosis on the upper GI study (control group). All children meeting the inclusion criteria mentioned above without gastroptosis on the upper GI study in a standing position were considered as the control group.
In most children, gastroptosis was not diagnosed at the time of the upper GI study. In 5 patients the radiologist did mention the downward displacement of the stomach; however, it had no consequences for further investigation or treatment.
The presence of delayed gastric emptying was recorded in the database, as well as information about gastric motility. Gastric emptying was defined as delayed when it was diagnosed through a gastric emptying scintigraphy or when it was delayed during the upper GI study according to the opinion of the radiologist. For scintigraphy, children had to eat a pancake with 10 MBq technetium 99 m hepatate II colloid according to the recipe of the recommendations of the Dutch Association of Nuclear Medicine (NVNG).12 Gastric emptying time was defined as delayed consistent with the same guidelines. Gastric motility was defined as impaired if it was reduced according to the opinion of the performing radiologist during the upper GI study.
Treatment and symptoms during treatment were recorded for all children. Conservative treatment was defined as expectant in combination with a diet. The period of follow-up was variable, depending on the time needed to set the treatment and the return of the children to the referring physician. The need for informed consent was waived by the institutional medical ethical review board.
Statistics
IBM SPSS Statistics version 21 (2012) was used for data analysis. Mann Whitney U-test was used to assess differences of age and BMI SDS between the group of children with gastroptosis and the control group. The significance of differences in incidences of GI symptoms, decreased gastric motility and delayed gastric emptying was assessed statistically by Fisher’s exact test. For all analyses, p<0.05 was used for statistical significance.
RESULTS
One hundred sixty-one children between the age of 6 to 18 years underwent an upper GI study in our university hospital between March 2010 and March 2015. Children with fundoplication in history (N=18) and children having a gastrostomy or an indication for gastrostomy (N=41) were excluded. Two children were excluded because the results of the upper GI study were the only available data. Another 40 children were excluded because the results of the upper GI study showed the stomach only in a supine position. Of the 60 children with an upper GI study in standing position that were included, 7 children had gastroptosis (11.67%).
Table 1 provides an overview of all 60 included subjects. The age of the children with gastroptosis (14.14±2.48) was significantly higher compared to the age in the control group (11.43±3.39; p=0.047). We found no significant differences in gender between the two groups (boys 28.6% vs. girls 43.4%; p=0.688).
| Table 1: Overview of Subjects Based on Upper GI Study. |
|
|
Gastroptosis (N=7) |
Non-gastroptosis (N=53) |
p-value |
| Gender |
Boy Girl |
2 (28.6%)
5 (71.4%)
|
23 (43.4%)
30 (56.6%) |
0.69 |
| Age |
(mean±SD) |
14.14±2.48 |
11.43±3.39 |
0.047* |
| BMI SDS |
(mean±SD) |
-0.83±1.11 |
0.12±1.69 |
0.06 |
| SD: standard deviation; BMI SDS: Body Mass Index Standard Deviation Score |
Overall, BMI SDS differed not significantly between the groups (p=0.06). However for girls, while BMI SDS was significantly lower in girls with gastroptosis compared to the control group (p=0.027), it was still within the normal range.
The indications for the performing of an upper GI study are shown in Table 2. For each child, more than one indication could be noted on the application form and these indications are demonstrated separately in the Table 2. There were no significant differences in indications.
| Table 2: Indications of the Barium Study in Children |
|
|
Gastroptosis (N=7) |
Non-gastroptosis (N=53) |
| Indications barium study |
Malrotation |
6 (85.7%) |
29 (54.7%) |
| Hiatal hernia |
0 (0%) |
19 (35.8%) |
| Gastroesophageal reflux |
1 (14.3%) |
13 (24.5%) |
| Gastric motility |
2 (28.6%) |
10 (18.9%) |
| Gastric emptying |
1 (14.3%) |
11 (20.8%) |
| Esophagus abnormalities |
0 (0%) |
16 (30.2%) |
| Passage/stenosis/stricture/obstruction |
4 (57.1%) |
11 (20.8%) |
| Gastrointestinal inflammation |
1 (14.3%) |
0 (0%) |
GI symptoms were compared between children with gastroptosis and the control group. Results of the analysis of the incidence of symptoms between the groups are shown in Table 3. None of the symptoms had a significantly higher prevalence in children with or without gastroptosis.
| Table 3: Symptoms in Children with and without Gastroptosis. |
|
|
Gastroptosis
N=7 (%) |
Non-gastroptosis,
N=53 (%) |
p-value |
| Symptoms |
| Symptoms of acid brash |
Present |
4 (57.1%) |
27 (50.9%) |
1.000 |
| Epigastric pain, discomfort |
Present |
7 (100%) |
36 (67.9%) |
0.18 |
| Constipation |
Present |
1 (14.3%) |
13 (24.5%) |
1.00 |
| Recurrent diarrhea |
Present |
0 (0%) |
6 (11.3%) |
1.00 |
| Nausea |
Present |
5 (71.4%) |
19 (35.8%) |
0.10 |
| Recurrent vomiting |
Present |
4 (57.1%) |
16 (30.2%) |
0.21 |
| Early satiety |
Present |
2 (28.6%) |
2 (3.8%) |
0.06 |
| Abdominal distension |
Present |
0 (0%) |
1 (1.9%) |
1.00 |
| Upper GI tract study |
| Decreased gastric motility |
Present |
3 (42.9%) |
1 (1.9%) |
0.004* |
| Delayed gastric emptying |
Present |
4 (57.1%) |
10 (18.9%) |
0.045* |
| *p<0.05 Significant |
Delayed gastric emptying was significantly more often present in children with gastroptosis than in the control group (p=0.045). Decreased gastric motility was identified significantly more often in the gastroptosis group (p=0.004). Of the 7 children with gastroptosis, 3 children had decreased gastric motility, of whom 2 also had delayed gastric emptying. One child with gastroptosis showed normal gastric emptying and normal gastric motility in supine position, but in a standing position there was no movement of food to the duodenum.
Treatment of the children with gastroptosis was symptomatically in all patients. Erythromycin was given in 4 patients, of whom one had to stop because of frequent vomiting as a side-effect, and one patient stopped because of the absence of improvement. Four children were treated with antacids, which reduced the symptoms in all patients, but not satisfactorily. In one patient with gastroptosis and delayed gastric emptying, symptoms recovered after treatment with Metronidazole for a Giardia lamblia infection. It is known that there is a relation between delayed gastric emptying and bacterial overgrowth, which could cause symptoms including diarrhea, bloating, malnutrition and weight loss.13,14 In one patient, symptoms disappeared entirely after a diagnostic laparoscopy, with detaching of an adhesion of the duodenum. In none of the other 5 subjects symptoms disappeared entirely within a year, despite of the treatment.
In the control group, treatment was also symptomatically, mostly conservative. There was no indication for surgery. Two patients were treated with Metronidazole for a Dientamoeba Fragilis infection. Symptomatic treatments of both groups are compared in Table 4. Erythromycin was used significantly more often in children with gastroptosis, which is likely due to the higher incidence of delayed gastric emptying in children with gastroptosis.
| Table 4: Management of Children with and without Gastroptosis. |
| Treatment |
Gastroptosis,
N=7 (%) |
Non-gastroptosis,
N=53 (%) |
p-value |
| Strictly conservative |
2 (28.6%) |
28 (52.8%) |
0.12 |
| Macrogol |
1 (14.3%) |
6 (11.3%) |
1.00 |
| Antacids |
4 (57.1%) |
16 (30.2%) |
0.35 |
| Erythromycin |
4 (57.1%) |
1 (1.9%) |
0.00* |
| *p<0.05 Significant |
DISCUSSION
This study demonstrates a higher incidence of decreased gastric motility and delayed gastric emptying in children with gastroptosis. GI symptoms, such as nausea, recurrent vomiting and acid brash, were not significantly more present in children with gastroptosis compared to the control group.
There are some mechanisms suggested to cause gastroptosis and GI symptoms. The downward displacement of the stomach in gastroptosis is suggested to be caused by relaxation or stretching of the muscles or by decrease of the muscle tone.1 However, in this study GI symptoms were not significantly different in children with gastroptosis compared to the control group. This may be caused by the fact that the symptoms of gastroptosis are non-specific and applicable to several other conditions.
In the literature, gastroptosis seems to be associated with delayed gastric emptying.1,2,3 In children, investigations to diagnose delayed gastric emptying are generally unreliable. Upper GI studies may demonstrate delayed gastric emptying, but sensitivity and specificity are low.15 In our study, in most of the cases the result of the upper GI tract study was used to define delayed gastric emptying, when gastric emptying scintigraphy did not take place. This could have introduced a false interpretation, because results of gastric emptying and gastric motility, as seen on an upper GI study, are dependent on the interpretation of the radiologist, as there are no reference values for gastric emptying and gastric motility in upper GI studies. However, as this is a retrospective study, the performing radiologists were not aware of this study at the time of the upper GI studies.
Delayed gastric emptying seems to be associated with gastroptosis, but the mechanism by which they are associated is unknown. Natsis et al3 suggested that gastroptosis could cause GI symptoms. They suggest that tubular structures are prone to kinking when internal organs are positioned low in the abdomen, which may cause a temporary obstruction of flow through these organs. This could lead to food remaining in the stomach, which can lead to GI symptoms.3 Gastric emptying time seems to be prolonged when the stomach lays in a lower position.3 Christianakis et al1 suggested that delayed diagnosis or misdiagnosis of gastroparesis could possibly secondarily lead to gastroptosis, due to decrease of the muscle tone.
In this study, children with gastroptosis had a higher prevalence of decreased gastric motility. The presence of decreased gastric motility was determined subjectively by the radiologist who initially evaluated the upper GI study. Decreased gastric accommodation is associated with symptoms including early satiety, bloating, epigastric pain, weight loss and nausea.5 Delayed gastric emptying, altered antroduodenal motility, and impaired gastric accommodation have been proposed to explain symptoms of functional dyspepsia.5,6,7,8,9 However, despite of the higher incidence of delayed gastric emptying and decreased gastric motility in the group of children with gastroptosis, there was no difference in the presence of GI symptoms. This may be caused by the fact that the symptoms of gastroptosis are non-specific and applicable to several other conditions.
In the past, the preferred treatment of gastroptosis was surgical, because gastroptosis was thought to cause gastric dysfunction.1,2 Barbat et al10 suggested that the only indication for an operative procedure is when there is actual obstruction, which is rare. Now-a-days treatment is symptomatically, because it is uncertain that gastroptosis itself leads to GI symptoms.
There are some limitations to this study. The study is retrospective, so it depends on the availability and reliability of data, which is especially tricky when studying a dynamic upper GI study with only a couple of images saved. Results of gastric emptying and gastric motility depend on the radiologist, as there are no standard values available for gastric emptying and gastric motility in children in upper GI studies. Another issue is the limited number of patients of the study group. Of the 161 patients who met the inclusion criteria, 60 were included from which seven could be diagnosed with gastroptosis. Still, to our best knowledge this is the largest study on gastroptosis in children.
All children underwent an upper GI study for an indication related to GI issues, which causes the control group to be unequal to the general population. However the upper GI study indications were similar in cases with or without gastroptosis.
Regarding this study and the literature, the diagnosis of gastroptosis does not seem to be relevant anymore. There is no particular medical treatment for children with GI symptoms with gastroptosis. Also surgery of gastroptosis has become obsolete. Treatment is symptomatically as it is not clear if symptoms are caused by gastroptosis itself. Gastroptosis could possibly be part of a disease complex; however, it has no relevance on its own.
We conclude that gastroptosis can be part of the complex of findings in patients with GI symptoms; however, it is not diagnostic for a particular disease, nor does it influence the treatment. Therefore, gastroptosis on an upper GI study is an irrelevant finding.
COMPLIANCE WITH ETHICAL STANDARDS
Conflicts of Interest: All authors declare no conflict of interest.
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Author’s Contributions: All authors are responsible for study design and writing and revising the manuscript.






