INTRODUCTION
Hepatitis B Virus (HBV) is an important worldwide cause of chronic liver disease in children. In endemic areas, perinatal transmission accounts for the majority of infections. After exposure, the risk of developing chronic hepatitis B (CHB) is highest during the first year of life (80-90%) and in children infected before the age of 6 years (30-50%).1 During childhood CHB is usually asymptomatic, which has led to the recommendation not to treat perinatally acquired CHB. But once adulthood is reached, the end result can be cirrhosis (3-5%), hepatocellular carcinoma (0.01-0.03%) and increased mortality (15-25%).1,2 A search for means to prevent this progression is important.
As persistent HBV replication is directly related to disease progression and complications,3,4 it may be reasonable to commence early preventative measures. Evidence from adult studies demonstrates that effective viral suppression slows disease progression,5,6 promotes histological regression and may lead to reversal of cirrhosis.7
Tenofovir Disoproxil Fumarate (TDF), a nucleotide analogue, is approved for the treatment of CHB in the United States. A randomized controlled trial showed that TDF treatment was safe and well tolerated in adolescents with a low incidence of adverse events, and with no resistant HBV mutations.8 TDF has not been assessed in pediatrics patients with perinatally acquired CHB. We examined the effects of TDF given to children and adolescent patients with perinatally acquired CHB over a 72-week period.
METHODS
Study Design
A retrospective chart review was performed of patients evaluated at the Digestive Diseases and Nutrition Center of the Women & Children’s Hospital of Buffalo between January of 2006 and September of 2013 who were diagnosed with CHB infection. The study was approved by the Children and Youth Institutional Review Board.
Study Population
Data from pediatric patients ages birth to 21 years of any gender and race with a diagnosis of CHB infection were analyzed. The majority of patients were immigrants or international adoptees from Southeast Asia and Africa residing in the United States. Patients were required to have a history consistent with perinatally acquired CHB, were not immunized against HBV, were treatment naïve and had hepatitis B surface antigen (HBsAg) positive serology for at least six months prior to treatment. Inclusion criteria included hepatitis B e antigen (HBeAg) positive serology, hepatitis B e antibody (anti-HBe) and hepatitis B surface antibody (anti-HBs) negative serology, elevated HBV DNA viral load (>105 copies/mL) measured by quantitative real time Polymerase Chain Reaction (PCR) and a liver biopsy prior to treatment. An upper limit of normal for alanine aminotransferase (ALT) was defined as >40 U/L. Patients were characterized as having immune active hepatitis (ALT >40 U/L) or being in the immune tolerant phase (ALT <40 U/L). Patients were excluded from the study if they were co-infected with hepatitis C virus or another hepatotropic virus or were taking medications considered to be hepatotoxic. Other recorded data included HBV genotype and baseline liver histopathologic findings.
Treatment and Follow-up Outline
Patients were required to have completed at least a 72-week course of therapy with a standard 300 mg daily dose of TDF. If patients were unable to swallow pills, an 8 mg/kg/day dose of TDF (40 mg/scoop of powder, maximum dose 300 mg/day) was prescribed. Serology, ALT and HBV DNA levels were recorded at baseline, week 4 and 8 after starting TDF and every 8 weeks thereafter until week 72. HBV DNA was measured using the COBAS AmpliPrep/TaqMan HBV Test (Roche Molecular Systems, Inc, CA, USA) with a lower limit of detection of <116 copies/mL.
End Points
The primary end point was an HBV DNA level below the lower limit of detection by 72 weeks of treatment. Response was compared between patients who had immune active hepatitis and those who were in the immune tolerant phase. Secondary end points included a normal ALT serum level, HBeAg and HBsAg clearance and antibody seroconversion by the end of the study.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism Software, version 6.0 (GraphPad Inc., La Jolla, CA, USA). Conventional descriptive statistics were performed for continuous variables and a student t-test was used to compare the two groups. Categorical values were compared using Chi-square test. A P value of <0.05 was considered statistically significant. Results are expressed as Mean values±Standard Error of the Mean (SEM). To determine serum viral load changes, HBV DNA measured in copies/mL was expressed logarithmically.
RESULTS
Patient Characteristics
A total of 55 cases were analyzed of which 26 were treated with TDF for at least 72 weeks (Figure 1). Of these, 14(54%) had immune active hepatitis and 12(46%) were in the immune tolerant phase. Most of the patients were of Asian race (73%) infected with genotype C HBV (69%). Other demographic and baseline laboratory characteristics are shown in Table 1.
Figure 1: Patient distribution.
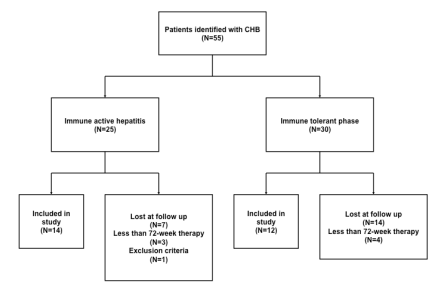
| Table 1: Demographics and Baseline Characteristics. |
|
Immune Active Hepatitis (n=14) |
Immune Tolerant Phase (n=12) |
Total (n=26) |
P value |
|
|
|
|
|
| Years of age, mean (range) |
12.6
(2-18) |
14.5
(9-19) |
13.5
(2-19) |
N/S |
| Sex, n(%) |
|
|
|
N/S |
| Male |
6(43) |
6(50) |
12(46) |
|
| Female |
8(57) |
6(50) |
14(54) |
|
| Race, n(%)* |
|
|
|
N/S |
| Asian |
8(57) |
11(92) |
19(73) |
|
| White |
1(7) |
0(0) |
1(4) |
|
| Black |
5(36) |
1(8) |
6(23) |
|
| HBV Genotype, n(%) |
|
|
|
N/S |
| A |
1(7) |
0(0) |
1(4) |
|
| B |
1(7) |
0(0) |
1(4) |
|
| C |
7(50) |
11(92) |
18(69) |
|
| D |
3(21) |
0(0) |
3(12) |
|
| E |
2(14) |
1(8) |
3(12) |
|
| Baseline serum ALT, U/L† |
|
|
|
<0.05 |
| Mean±SEM |
86.9±10.2 |
24.3±2.1 |
58.0±8.3 |
|
| (range) |
(43-173) |
(12-36) |
(12-173) |
|
| Baseline HBV DNA
log10 copiel/mL |
|
|
N/S |
| Mean±SEM |
9.03±0.09 |
8.96±0.06 |
9.01±0.06 |
|
| (range) |
(8.78-9.09) |
(8.80-9.69) |
(8.78-9.91) |
|
| Baseline Serology |
|
|
|
N/S |
| HBsAg positive, n(%) |
14(100) |
12(100) |
26(100) |
|
| HBeAg positive, n(%) |
14(100) |
12(100) |
26(100) |
|
| Baseline Histology‡ |
|
|
N/S |
| Mean inflammatory score |
1.6 |
1.1 |
1.28 |
|
| (range) |
(1-3) |
(1-2) |
(1-3) |
|
| Mean fibrosis score |
1 |
0.7 |
0.8 |
|
| (range) |
(0-2) |
(0-1) |
(0-2) |
|
| Abbreviations: n: Number; N/S: Not Significant; SEM: Standard Error of Mean; U/L: Units/Liter.
*Race was self-reported by patient.
†ALT above or below upper limit of normal (40 U/L).
‡ Based on Batts-Ludwig scoring system |
Baseline Histopathology
All treated patients had a liver biopsy prior to starting therapy with TDF. A single pathologist from our institution analyzed the biopsies. Batts-Ludwig scoring system was used for histological assessment.9 Overall, patients showed a mean inflammatory score of 1.28 (range 1-3) and a mean fibrosis score of 0.8 (range 0-2). When compared there was no difference in the mean inflammatory or fibrosis scores between those who had immune active hepatitis or those in the immune tolerant phase (P=0.17).
Virologic Response
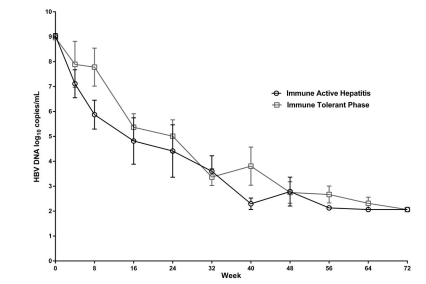
Patients showed a mean serum HBV DNA level of 9 log10 copies/mL before starting therapy with TDF. At week 40, the overall mean HBV DNA level decline was 5.9 log10 copies/mL. Individual group analysis demonstrated that the mean HBV DNA level in the immune active hepatitis group was reduced by a mean of 6.7 log10 copies/mL at 40 weeks compared to 5.1 log10 copies/mL in the immune tolerant phase group. By week 72, 19/26(73%) of patients had HBV DNA levels below the lower limit of detection, 11(42%) from the immune active hepatitis group and 8(31%) from the immune tolerant phase group. There was no difference in virologic response between the two study groups (P=0.61) (Figure 2).
Figure 2: Virologic response. Mean reduction and comparison in HBV DNA levels (log10 copies/mL) between immune active hepatitis and immune tolerant phase patients over a 72-week treatment period with TDF.
Biochemical Response
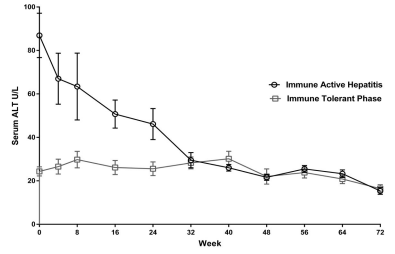
At baseline, patients in the immune active hepatitis group showed a mean ALT value of 86.9 U/L. As preconditioned, mean ALT values were within the normal range in the immune tolerant phase group. During the first 32 weeks of therapy, ALT values in 8/14(57%) patients with immune active hepatitis reached normal levels. By 72 weeks, all patients with immune active hepatitis achieved normal ALT levels (Figure 3). No breakthrough ALT elevations were seen in the immune active hepatitis or immune tolerant phase patients.
Figure 3: Biochemical response. Mean ALT (U/L) reduction and normalization in immune active hepatitis patients.
Serologic Response
All patients were HBeAg positive and anti-HBe negative prior to starting TDF therapy. Across both treatment groups, HBeAg clearance was seen in 42% of patients and anti-HBe seroconversion occurred in 35%. None of the patients had HBsAg clearance or anti-HBs seroconversion.
DISCUSSION
Data from this 72-week retrospective analysis in children and adolescents with perinatally acquired CHB suggests that TDF therapy is effective in decreasing HBV DNA serum levels. This effect was seen in all patients whether they had elevated or normal ALT levels at the beginning of therapy. Similar to our results, Murray, et al. found that TDF antiviral efficacy was high regardless of baseline ALT values.8 However, we were able to assess baseline liver histology and found no difference in either inflammation or fibrosis between patients who had normal ALT levels and those who had elevated ALT levels. This is an important observation as it calls into question the notion of “immune tolerance” as the immune tolerant phase group had as much histologically demonstrated hepatic inflammation and fibrosis, as did the immune active hepatitis group. In this study we were able to compare virologic response between these two groups of patients. The fact that there was no difference in either histology or outcomes suggests that assignment of children to one of the two groups with the intent of deciding whether or not treatment is indicated is of no clinical value.
The rate of ALT normalization was high in patients with immune active hepatitis and no breakthrough elevations were seen in either group during the study period. Patients studied in this analysis all had a high viral load and were HBeAg positive at baseline. A 3-year study that analyzed TDF monotherapy on adult patients with CHB showed that it was effective in lowering and maintaining normal levels of ALT, whether they were HBeAg positive or negative.10 Gordon, et al. demonstrated that the rate of ALT normalization was rapid in adults regardless of baseline viral load.11 In pediatrics, our findings suggest that TDF was effective in lowering ALT levels in patients with a high viral load at the beginning of therapy. Whether baseline HBeAg status is a determining factor for biochemical response is unknown. It is known, however, that viral load is a risk factor for the development of complications associated with HBV infection, such as hepatocellular carcinoma12 and that lowering the viral load is a desirable goal.
The overall rate of serologic response during this 72-week analysis showed that a considerable number of patients had HBeAg clearance and anti-HBe seroconversion. This suggests enhanced immunologic activity against HBV that might coincide with TDF induced reduction in HBV DNA levels. During the analyzed period, no patients had HBsAg clearance or seroconversion, signaling elimination of virus. It is known that the HBV DNA is sequestered in the nucleus of the host hepatocyte as covalently closed circular DNA (cccDNA) where it is difficult but not impossible to eliminate.13 It appears that TDF suppresses viral replication, but additional therapy may be needed to cure the infection.
Our single center study offers new and important information. For instance, we were able to assess TDF response in pediatric patients infected with HBV genotype C, one of the most common forms encountered in the United States,14 as well as in patients as young as 2 years of age. Our study does not answer several related questions about perinatally acquired HBV. First, does viral suppression indeed affect the long-term progression of the disease, improving outcome? Follow up studies are needed to answer this question. Second, for whom should we consider TDF treatment? Given that there area certain number of cases of perinatally acquired HBV that will seroconvert to anti-HBs positivity each year, is it possible to identify these individuals and avoid treating them? How long should we treat? Is this an entity that requires indefinite treatment to keep the infection in check? Is there truly a difference between immune active and immune tolerant phases?
Our study population was predominantly refugees from Southeast Asia and Africa and as such there was usually a language and cultural barrier to overcome. In addition TDF therapy was offered only as option. This accounts for the high dropout rate (55 subjects identified but 26 completing 72 weeks of therapy). Long-term follow up studies are warranted to confirm our observations and to begin to answer the questions, which our study raises. Although all patients had a liver biopsy prior to starting therapy, no end of treatment biopsy was performed to assess histologic response.
We conclude that TDF effectively decreases HBV load in children with perinatally acquired CHB, whether they have immune active hepatitis or are in the immune tolerant phase. TDF also normalizes ALT, promotes HBeAg clearance, and anti-HBe seroconversion. We question the relevance of designating groups such as immune active hepatitis and immune tolerant phase based on beginning of therapy ALT values since both groups had similar levels of circulating HBV, liver inflammation and fibrosis and, response to TDF treatment.
DISCLOSURE
The authors have no financial disclosures or conflicts of interest to declare.
CONSENT
The research work for the manuscript entitled “Tenofovir Disoproxil Fumarate Treatment for Pediatric Patients with Perinatally Acquired Chronic Hepatitis B” did not require parental consent or patient asset since this was a retrospective chart review.
ACKNOWLEDGEMENT
The authors thank Dr. Changxing Ma for his assistance in the statistical analysis of the data.








