INTRODUCTION
Biofilms are a significant threat to public health. Biofilms can form on surfaces such as medical devices, wastewater pipes, human tissue, and stagnant water bodies. The properties of the bacteria in the biofilm influence their strength of attachment and how strong the biofilm is.1 The primary reason biofilms are hard to combat the bacterial interactions that take place within the biofilm. Bacteria survive by forming biofilms on surfaces. The structured formation of bacteria imparts resistance to chemicals, phagocytosis, and antibiotics to the bacteria in the biofilm.2 The bacteria grow in structured communities or biofilms, and an extracellular matrix surrounds the cell conglomerates. Quorum sensing (QS) is a form of cell-to-cell communication that plays a crucial role in the formation of biofilms. Many bacteria can regulate their activities and physiological processes through QS. The bacteria communicate by releasing, recognizing, and responding to specific signal molecules. This feature of bacteria allows them to coordinate their functions and detect the density of the bacteria surrounding them.3 The QS controls biofilm formation to increase cooperation and stabilize the biofilm. Because of the increased interactions between the bacterial cells, biofilms become hard to destroy. Although each bacterium can still be killed using antibiotics, the aggregates of cells in the biofilm are not susceptible to drugs. The electrical charges on the biofilm prevent it from being destroyed by antibiotics. Furthermore, the cells located deep in the biofilm do not have access to a rich source of nutrients and, if required, oxygen. In turn, the cells grow slowly, making them less susceptible to antibiotics, which work best on actively dividing cells. Additionally, the biofilms are impervious to destructive chemicals like ethanol. Biofilms, when considering a medical perspective, commonly grow on medical devices, and in human tissues like skin wounds because of their adherence properties. Their impermeability to commonly used methods of microbial destruction and their prevalence in everyday life, combined with their significant threat to public health, depicts the importance of researching methods to combat the growth of biofilms. The project aims to combat the growth of biofilm using the medicinal plant extract of Taraxacum officinale and probiotics. The Taraxacum officinale extract has antibacterial properties that can inhibit QS, thereby limiting the growth of biofilm.4 This extract comes from the dandelion plant, which has been used in Native American, Arabic, and traditional Chinese medicine.5 The extract comes from a crude extract of the dandelion root.6 The compounds of hexane and ethyl acetate in the Taraxacum officinale extract prove to have efficient antimicrobial properties.7 Furthermore, the bacterial cells in the biofilm aggregate do not have resistance to the extract. The other treatment studied is a probiotics solution of Lactobacillus acidophilus, Bifidobacterium lactis, and Bifidobacterium longum. Probiotics can break apart biofilms. The probiotics also weaken the cells in the biofilms, thereby slowing the growth or expansion of the biofilms. The bacteria in probiotics are engaged in competitive microbial and metabolic interactions with the other bacteria. The direct result of this is the production of antimicrobial compounds or molecules that have bactericidal properties..8 These two methods of destruction are possible solutions to the threat the biofilms pose. If these research methods prove to be effective, they will be instrumental in combating the biofilms in tissues and on medical devices (which lead to further complications). The hypothesis for this research aims to develop methods of bacterial biofilm destruction, of which efficient methods do not currently exist. The Taraxacum officinale extract and the probiotics are expected to be more effective in combating the biofilm than the traditional antibiotics or other methods of bacterial destruction. The objective and research question of this comparative study is to research the potential and effectiveness of treatments to combat the growth of biofilm formed by E. coli9 and E. aerogenes by using two approaches: medicinal plant extract of Taraxacum officinale and probiotics.
METHODOLOGY
The area of study is the destruction of bacterial biofilms, a topic that requires further research because of the intensity of biofilm infections. The study design is a comparative study in which the effectiveness of probiotics, the Taraxacum officinale extract, and antibiotics are compared in their ability to destroy bacterial biofilms. Data was analyzed by measuring the diameters of the zones of inhibition produced by the treatments in the biofilms.
Procedure
Step 1: Agar preparation and plate preparation
• Twenty grams (on an electronic balance on a weighing boat) of tryptic soy agar (this type of agar is essential for these bacteria to grow) was measured and combined with 500 mL of distilled water in a conical flask.
• A magnetic stir bar in the flask was used on top of the hot plate to help mix the agar.
• Agar solution was autoclaved. The mixture was cooled down and poured into the agar plates. The Petri dishes were labeled on the bottom.
Step 2: Streaking and preparing diffusion disks
• After all the plates have been streaked, place the filter paper disks into their respective treatments. • Place 18 disks into the Taraxacum officinale extract and place 18 disks into the probiotic solution of one capsule of probiotics and 25 mL of distilled water.
• Use tweezers to place 3 disks in a triangular shape, about 3 centimeters away from each other.
• Tap the disks in the middle with tweezers to ensure that they are in contact with the streaked plates.
• Do not place the disks onto plates that are under the control group.
• After all the plates have been streaked, and the disks have been placed, incubate them for 24 to 48-hours at a temperature that is the average of the optimal temperatures (35 °C) for growing the two types of bacteria: E. coli and E. aerogenes.
• After the incubation time, remove the plates from the incubator.
• Measure the zones of clearance by using a ruler to find the diameters of the rings formed around the diffusion disks on the plates.
Record all data and analyze the results.
Biosafety Level
This experiment used non-pathogenic bacteria that can be used in the setting of a high school lab. Proper safety measures and personal protective equipment such as goggles, aprons, closed-toe shoes, long hair tied back, and no loose-fitting clothes were worn in the lab. A regulated research institution was not needed to complete this lab.
Data
Three trials were conducted in this experiment, for each of the two bacteria, across the two different treatments of Taraxacum officinale extract and the probiotic mix. There was also one set of control (one Petri dish with each bacterium, with non-treated disks) for each of the three trails. Each trial involved testing the bactericidal properties of Taraxacum officinale extract and the probiotic mix against the biofilm. The diameters of the zones of inhibition for the plates with the antibiotic disks was measured after 48 hours. If a zone of inhibition forms around the disk, it means that the disk with the extracts of Taraxacum officinale extract and the probiotic mix had bactericidal properties. The diameters of the zones of inhibition was measured using a millimeter scale.
Tables 1 and 2 show the data collected for this research study over three trials and two treatments for each of the 2 bacteria types.
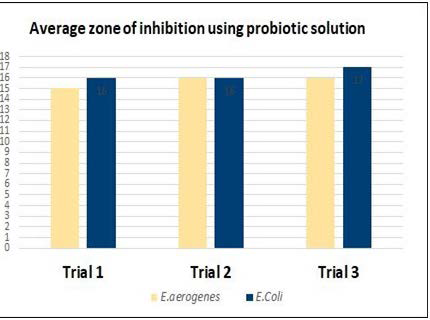
| Table 1. Measurement of the Diameter of the Zones of Inhibition in the Three Trials with Probiotics Treatment for Each Disk for Each Bacteria |
|
Bacteria
|
Trials |
Zone of Inhibition (in mm) (average for the three disks)
|
| E. aerogenes |
Trial 1
|
15
|
| E. aerogenes |
Trial 2
|
16
|
| E. aerogenes |
Trial 3
|
16
|
| E.Coli |
Trial 1
|
16
|
| E.Coli |
Trial 2
|
16
|
| E.Coli |
Trial 3
|
17
|
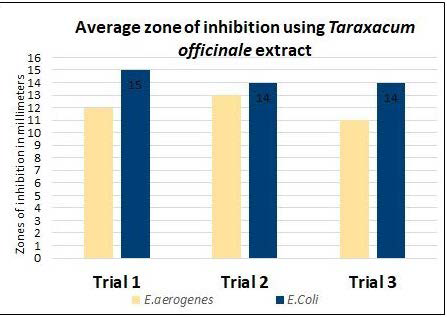
| Table 2. Measurement of Zones of Inhibition in the Three Trials of Taraxacum officinale for Each Disk for Each Bacteria |
|
Bacteria
|
Trials |
Zone of Inhibition (in mm) (average for the three disks)
|
| E. aerogenes |
Trial 1
|
12
|
| E. aerogenes |
Trial 2
|
13
|
| E. aerogenes |
Trial 3
|
11
|
| E.Coli |
Trial 1
|
15
|
| E.Coli |
Trial 2
|
14
|
| E.Coli |
Trial 3
|
14
|
Graphs 1 and 2 depict the average zones of inhibition for each bacteria over the three trials.
Graph 1. The Values after Treatment with a Probiotic Solution
Graph 2. The Average Zones of Inhibition for Each Bacteria after Treatment with Taraxacum officinale
The antibiotic resistance Table 3 shows the values of the zones of inhibition in millimeters for the two antibiotics traditionally used for each bacteria type. Sulfamethoxazole-trimethoprim is traditionally used as an antibiotic against E.coli, whereas Ampicillin is used as an antibiotic against E. aerogenes.
| Table 3. Traditional Antibiotic Resistance Table10 |
|
Antibiotic
|
Disc Code |
Resistrant |
| (Antimicrobial Agent) |
(< or=mm)
|
|
Ampicillin
|
AM |
11 |
| Sulfamethoxazole-trimethoprim |
SXT |
10
|
Tables 4 and 5, Figures 1 and 2 are the tables and graphs from the statistical tests conducted from this study. The data represented on these figures and graphs show the statistical significance of the results from this study.
Figure 1. Tukey HSD / Tukey Kramer
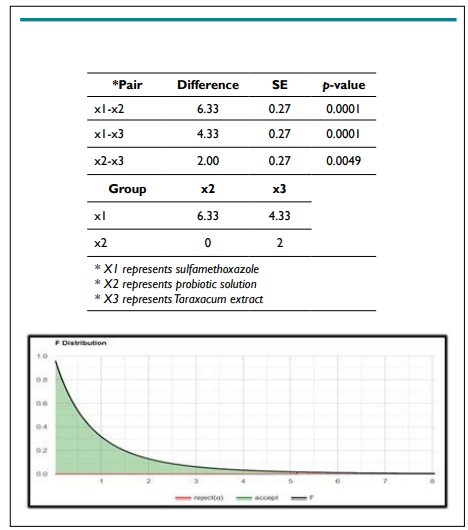
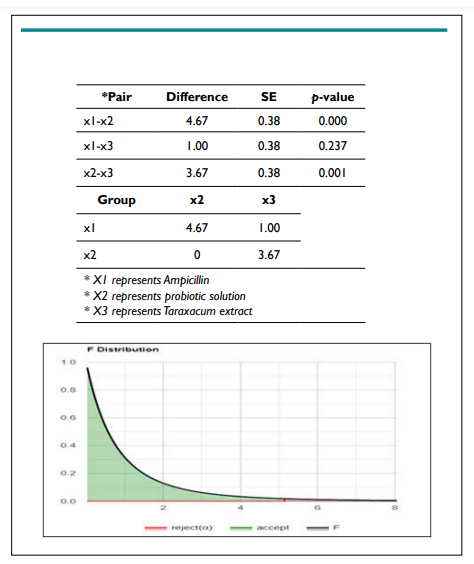
Figure 2. Tukey HSD / Tukey Kramer
| Table 4. ANOVA Test of the Three Treatments for E.coli (SXT, Probiotic Solution, and Tarazacum officinale) |
|
Source
|
DF |
Sum of Square |
Mean Square |
F Statistic |
p-value
|
| Groups (between the groups) |
2
|
62.89 |
31.44 |
141.50 |
0.0001
|
| Error (with groups) |
6
|
1.33 |
0.22
|
|
|
| Total |
8
|
64.22 |
8.03
|
|
|
| Table 5. ANOVA Test of the Three Treatments for E.aerogenes Ampicillin, Probiotic Solution, and Taraxacum officinale |
|
Source
|
DF |
Sum of Square |
Mean Square |
F Statistic |
p-value
|
| Groups (between the groups) |
2
|
36.22
|
18.11
|
40.75
|
0.0033
|
|
Error (with groups)
|
6 |
2.67 |
0.444 |
|
|
|
Total
|
8 |
38.89 |
4.86
|
|
|
RESULTS
Table 1 gives the zones of inhibition for the three trials that used disks with probiotics for both E. coli and E. aerogenes. Table 2 gives the zones of inhibition for the three trials that used disks with Taraxacum officinale. The diameters for the zones of inhibition formed by the diffusion disks was measured. This gives an insight into the effectiveness of each treatment applied to biofilm. The size of inhibition is related to antimicrobial activity. A standard t-test was run to compare the results of two groups of means for each bacteria. For E. aerogenes, the probiotic solution was more effective than dandelion extract with a t-value of 5.5 and a p-value of 0.0003, which is less than a p-value of 0.05. For E. coli, the probiotic solution was more effective than dandelion extract with a t-value of 6.5, and the p-value is less than 0.05.
Data from the trials were compared to traditional antibiotics. For E. coli, Sulfamethoxazole-trimethoprim is the standard antibiotic used for comparison, and for E. aerogenes, Ampicillin was the standard antibiotic. Zones of inhibition for these antibiotics (resistant values) were obtained from standardized guideline charts.
One Way ANOVA Test, Using F distribution df(2,6) (right-tailed) for E. coli:
The results from this test gave a p-value of 0.0001 (p<0.05), which signifies that the null hypothesis (H0) should be rejected, and the<0.05), which signifies that the null hypothesis (H0) should be rejected, and the alternative hypothesis for this experiment must be accepted.
One Way ANOVA Test, using F distribution df(2,6) (right-tailed) for E. aerogenes:
The results from this test gave a p-value of 0.0033 (p<0.05), which signifies that the null hypothesis should be rejected, and the alternative hypothesis for this experiment must be accepted.
The Tukey HSD Test
The Tukey HSD test for E. coli showed that the means of (antibiotics-probiotic solution), (antibiotics-Taraxacum extract), and (probiotic-Taraxacum extract) are all statistically significant.
The Tukey HSD Test
The Tukey HSD test for E. coli showed that the means of (antibiotics-probiotic solution), (antibiotics-Taraxacum extract), and (probiotic-Taraxacum extract) are all statistically significant.
DISCUSSION
The significance of this study is rooted in the fact that bacterial biofilms currently have no effective means of being destroyed. Because of how the biofilms manifest in the human body and in hospitals, they proliferate wound infections and lead to a rise in nosocomial infections. The results of this study suggest that the Taraxacum officinale extract and the probiotics solution are potential bacterial biofilm destruction methods. The implications of this study are potential methods of destroying the biofilms that cause infections in patients. One of the limitations of this study is that only two types of bacterial biofilms were studied, so it can not be said for certain that these methods of destruction could prove to be effective against all bacterial species. Some future recommendations for this study are to increase the number of trials and bacterial species studied.
CONCLUSION
The results above show that both treatments are options for combating biofilm growth, signified by their bactericidal properties. The zones of inhibition are used to measure the ability of a substance to inhibit microbial growth. In other words, it measures the ability of an antimicrobial agent to inhibit the growth of microorganisms. A larger zone of inhibition is indicative of the organism’s susceptibility to the treatment. Results show that the probiotics treatment was more effective in destroying the biofilms than the Taraxacum officinale extract. However, the Taraxacum officinale extract was more effective than the respective traditional antibiotic treatment for each bacteria.
Both treatments demonstrated potential bactericidal properties against biofilms regardless of the QS and the chemical interactions within or on the surface of the biofilms. These two methods of destruction are possible solutions to the threats the biofilms pose.
CONFLICTS OF INTEREST
This research did not have any research funding. This research had no conflict of interest because all work was done by the sole author.









