INTRODUCTION
Sugammadex introduction to the anesthetic community has been referred to as “a milestone”,1 “a revolutionary approach”,2 and “a turning point in the practice of anesthesiology”.3 Anesthesiologists were quite excited about this new medication. Indeed, no truly unique or revolutionary medications have been developed in the 20-years since the introduction of sugammadex. Anesthesiologists also needed a medication that could quickly restore neuromuscular blockade due to the many drawbacks of the anticholinesterases that are frequently employed as reversal agents following the injection of neuromuscular blockers.4
Sugammadex was unintentionally found, like many other important discoveries. A pharmaceutical chemist named Anton Bom was looking for a fresh solvent that might improve rocuronium bromide’s solubility. Instead, he was the first to realize that the use of cyclodextrin as a solubilizing agent over an extended period of time reduces the effectiveness of rocuronium.4
PHARMACOLOGY
Sugammadex is an selective relaxant binding agents (SRBA) that is not connected chemically or pharmacologically to acetylcholinesterase inhibitors. Reversal with sugammadex does not induce the cardiovascular side effects that are often seen with acetylcholinesterase (AChE) inhibitors since its mode of action is different than that of AChE Inhibitors.5 This substance encapsulates the neuromuscular blocking drug, and the complex is eliminated by kidney rather than increasing the quantity of acetylcholine at the neuromuscular junction (NMJ). Sugammadex blocks the interaction between the steroidal neuromuscular blocking drug and the nicotinic receptor at the NMJ by acting as a pharmacologic “sink” (i.e., an agent capable of absorbing or eliminating a substance from a system). The four hydrophobic rings of the steroidal neuromuscular blocking drug fit snugly inside the concentric doughnut shape of the modified gamma-cyclodextrin, generating a tight 1:1 complex with the aminosteroid-based relaxant.1 This cyclodextrin was then altered with several acidic functional groups to create an electrostatic connection with the positive nitrogen of the blocker, which would help with binding.2 Sugammadex does not affect the effects of the benzylisoquinolinium class of non-depolarizing drugs because of its structural specificity. Sugammadex has a high degree of selectivity in its binding to rocuronium because it was first created for that substance.5 Vecuronium can be effectively resisted even when other aminosteroid-based substances do not attach with the same affinities.1 To reverse pancuronium, sugammadex dosages may need to be increased. Due to the fact that endogenous steroids are not quaternary ammonium compounds, it would be reasonable to assume that their binding affinity for this novel substance would be low.3
MOLECULAR STRUCTURE
Sugammadex is a cyclodextrin that has a hydrophilic outer shell and a lipophilic interior. Sugammadex sodium has the chemical name 6-perdeoxy-6-per (2-carboxyethyl) thio-cyclodextrin sodium salt, which has the chemical formula C72H112O48S8. “Su” refers to sugar, and “gammadex” refers to the structural molecule gamma-cyclodextrin. Cyclodextrins are cyclic oligosaccharides composed of several glucose units linked by α-1,4 glycosidic bonds using cyclodextrin glycosyltransferase.6 The three most common cyclodextrins are α-, β-, and γ-cyclodextrin, which are composed of 6, 7, and 8 glucose units, respectively. Sugammadex is a γ-cyclodextrin derivative, meaning it is composed of 8 glucose units.
The natural state of this gamma cyclodextrin has been changed by putting eight carboxyl thio-ether groups on the sixth carbon. These additions make the cavity bigger, so the larger structure of the aminosteroid muscle relaxant molecule (e.g., rocuronium) can fit inside it better. This modification strengthened the structural stability of the four rocuronium hydrophobic steroidal rings contained within the hydrophobic cavity. Eight negatively charged carboxyl groups are inserted into the structure, which achieves two goals. First, the hole is kept open, and second, its structural integrity is maintained because the repelling forces of the negative charges keep the side chains of propionic acid from becoming disorganized.6 The inclusion of these negatively charged carboxyl groups enhances the rocuronium quaternary nitrogen’s electrostatic affinity even further. The three-dimensional form resembles a hollowed-out cone or a doughnut (Figure 1).7
Figure 1. Chemical Structure of Sugammadex
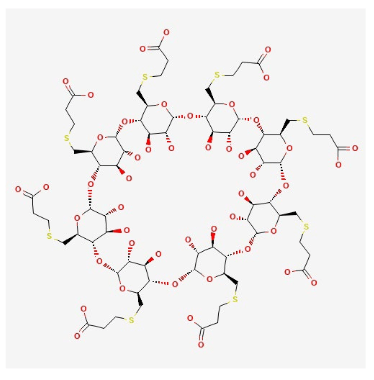
Source. https://pubchem.ncbi.nlm.nih.gov/substance/13526207437
PHARMACOKINETICS
Sugammadex binds intravascular rocuronium almost instantly after intravenous administration in the central compartment. As a result of the concentration gradient, rocuronium moves from the peripheral compartment (NMJ) to the central compartment, where it is also encapsulated by sugammadex. Consequently, muscle activity and neuromuscular communication recover rapidly. Therefore, a greater sugammadex dosage is preferable than a smaller one in order to reduce the plasma level of free rocuronium.8 Sugammadex pharmacokinetics show a linear, dose-dependent relationship in the dosage range of 0.1-16 mg/kg. Sugammadex has an elimination half-life ranging from 100 to 150-minutes. It is not digested by the body and is almost completely excreted by the kidneys, which have an average glomerular filtration rate of between 75 and 120 mL/min. In a study using radioactively tagged sugammadex, healthy volunteers eliminated 70% of the dosage in six hours and over 90% in twenty-four hours, demonstrating the drug’s quick elimination from the body.9 Based on other studies, a 24-hour excretion rate of 59-80% is projected.8 From a therapeutic point of view, it is important that the hepatic biotransformation and eventual clearance of rocuronium through biliary excretion are moved to a completely new (liver-independent) renal pathway when sugammadex is present. Consequently, patients with renal insufficiency require extra care. Although the mechanism of action of sugammadex is independent of renal perfusion10 and end-stage renal disease (ESRD) only eliminates 29% of the sugammadex-rocuronium molecules in a 72-hour period. Sugammadex is not advised for patients with low creatinine clearance (30 mL/min) or those requiring dialysis.10 Despite the fact that no cases of recurrence of neuromuscular block have been reported in renal failure patients receiving 2 mg/kg sugammadex at a return of T2 (2nd twitch of train-of-four (TOF) stimulation). This is due to the fact that the studies on this subject were not sufficiently powered for safety. Sugammadex, on the other hand, can be dialyzed using the proper dialysis filter.11
CLINICAL USE/DOSAGE
Varying sugammadex dosages are recommended depending on the muscle relaxant used and the degree of the neuromuscular block at the time of reversal. The dosages must be able to accelerate recovery from the neuromuscular block and obtain a TOF ratio of 0.9 in a median of three minutes. At any dose, it is not advised to quickly reverse a vecuronium-induced neuromuscular block.11
PHARMACODYNAMIC PROPERTIES
Mechanism of Action
Rocuronium, an aminosteroid non-depolarizing neuromuscular blocking drug that is often used during anesthesia in several nations, was especially chosen for Sugammadex’s formulation.12 Sugammadex has eight side chains, which lengthen the central cavity to completely enclose rocuronium. The carboxyl groups at the end of each chain are negatively charged. This makes it easier for the positively charged nitrogen atom in rocuronium to stick to the negatively charged nitrogen groups in the chains.13 Sugammadex also reverses the action of vecuronium, since rocuronium and vecuronium have a structural resemblance.14
Sugammadex has a different mode of action than other neuromuscular reversal drugs that are currently present in the market.15 Sugammadex binds to plasma levels of free rocuronium or vecuronium in a specific manner. The neuromuscular blockade caused by these medications is reversed because of sequestration, which lowers the quantity of rocuronium or vecuronium that is accessible to bind at active sites, including nicotinic receptors at the NMJ. The resultant sugammadex-rocuronium compound is inert16 and is excreted from the body in accordance with sugammadex pharmacokinetic features (Figure 2).
Figure 2. A. Schematic of a Sugammadex Molecule Encapsulating a Rocuronium Molecule. B. A Space-filling Model of the Sugammadex Sodium Molecule in the Same Orientation
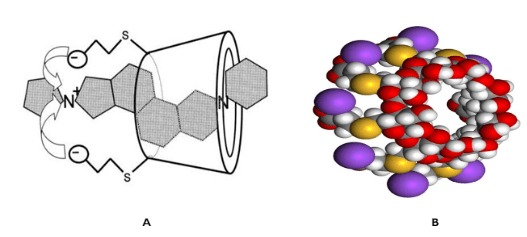
Reversal of Neuromuscular Blockade
Sugammadex 0.5-8.0 mg/kg dose-dependently reverses the neuromuscular blockade caused by rocuronium with a dose range of 0.6-1.2 mg/kg or vecuronium 0.1 mg/kg when administered at a post-tetanic count of 1-2 or the reappearance of T2 in surgical patients. Sugammadex is effective in a dose-dependent manner (range 0.1-16.0 mg/kg) in the immediate reversal of rocuronium 0.6-1.2 mg/kg during propofol-induced anesthesia.17 In phase II dose-finding studies, several individuals had persistent neuromuscular blockade at sugammadex dosages less than indicated (2 mg/kg).18 Therefore, the suggested dose for reversal of neuromuscular blockade (NMB) is more than 2 mg/kg (Table 1).
Cardiovascular Effects
Sugammadex may trigger adverse effects such as QTc prolongation. The QT interval is the amount of time it takes for the ventricles of the heart to depolarize and repolarize. The QTc interval is the corrected QT interval for heart rate. The risk of potentially fatal arrhythmias like Torsades de Pointes rises when the QTc interval is abnormally long. However, it is important to note that the occurrence of these side effects is low, and more research is needed to determine its safety.
In two “thorough QT/QTc studies” with 146 awake, healthy volunteers, sugammadex alone, with rocuronium, or with vecuronium was not linked to QTc interval prolongation. The largest mean difference to placebo in QTcI was 1.8 or 2.1 msec with sugammadex 4 mg/kg (a recommended dose), and 2.8 or 2.9 msec with sugammadex 32 mg/kg (a higher than recommended dose).12
Corrected QT (QTc) prolongation is strongly linked to traditional reversal with neostigmine. Sugammadex, on the other hand, has a little impact on QTc19 but a moderate impact on QT prolongation.20
Indication
Sugammadex is used in the operating theater, remote anesthesia, and the critical unit (ICU) to restore NMB. It is commonly used in developed countries as an non-depolarizing muscle relaxant (NDMR) reversal agent (mainly rocuronium) in bariatric surgery for obesity with a body mass index (BMI)>30. Sugammadex is the preferred reversal agent for patients with compromised respiratory reserve (asthma and chronic obstructive pulmonary disease (COPD) who are undergoing surgery, as opposed to neostimine, which has adverse effects including bronchospasm, bronchoconstriction, and laryngospasm. Finally, it is safe to use in the group of elderly and pediatric (patients older than 2-years) patients who are having surgery.
Contraindications
Sugammadex allergies are the sole absolute contraindication. Patients with 1. bleeding issues, 2. compromised renal function, and 3. who are taking toremifene or fusidic acid need to be treated with extra caution.11
ADVERSE REACTIONS
Following the administration of sugammadex, nausea and vomiting, headaches, itching, and dysgeusia are the most frequently reported side effects.21
Sugammadex administration has been associated with bradycardia and occasional occurrences of cardiac arrest.22 Bradycardia is a recognized adverse effect of sugammadex, and the manufacturer made this apparent in the package insert and subsequent briefing notes. Despite reports of QT prolongation after sugammadex treatment, the cause of bradycardia associated with sugammadex is unknown.20 Anticholinergic medications, such as atropine, have been used to treat severe bradycardia and prevent cardiac arrest. It is advised that whenever sugammadex is used, vasoactive medications like epinephrine and atropine be on hand. Sugammadex administration has been associated with bronchospasm, although the frequency seems to be fairly low. A few cases have been reported of residual NMB after reversal with sugammadex.
Specific Disease and Sugammadex
Sugammadex has been successfully introduced for patients with neuromuscular diseases or those at risk for postoperative respiratory dysfunctions, such as Duchene muscular dystrophy, Amyotrophic Lateral Sclerosis, and Myasthenia Gravis.23 In such condition, sugammadex provides rapid and reliable reversal from NMB. After its use for temporary muscle relaxation, the recovery time for spontaneous breathing and the side effects of electroconvulsive therapy (ECT) were both reduced.24 As a result, sugammadex is increasingly being used by patients with serious comorbidities as a safe NMB reversal medication.25
Change in Practice after Sugammadex
The ideal neuromuscular blocking drug would have no clinically relevant side effects, quick onset, and offset times, and transient, non-cumulative, non-depolarizing neuromuscular activity.26 In comparison to the most commonly used non-depolarizing drugs (the benzylisoquinolone drugs atracurium and cisatracurium, as well as the steroid-based drugs rocuronium and vecuronium), rocuronium has the quickest onset of action (1-minute with 1.2 mg/kg or 2-minutes with lower doses), and a midrange duration of action (37-minutes with 0.6 mg/kg). Suxamethonium chloride acts rapidly (less than 1-minute at 1 mg/kg) and quickly wears off (5 to 7-minutes at 1 mg/kg).27 The anticholinesterases, of which neostigmine is the most often utilized, have been the most regularly used neuromuscular blockade reversal medications for more than 30-years.15 These substances have no impact on the metabolism or clearance of neuromuscular blocking substances; instead, they work by preventing the breakdown of acetylcholine, increasing the quantity of acetylcholine at the neuromuscular junction. The difficulty of these medications to restore profound neuromuscular blockade and the requirement for concomitant anticholinergic agent delivery to reduce side effects are major drawbacks. Furthermore, because of anticholinesterase has a shorter duration of action than neuromuscular blocking drugs, residual paralysis may occur.3
In order to selectively undo the neuromuscular blockade brought on by rocuronium or vecuronium, sugammadex is the first selective relaxant binding drug to be advised for use in anesthetic treatment. Sugammadex has a distinct mode of action when compared to prior reversal medicines since it inactivates the neuromuscular blocking drug and has a high affinity and specificity for rocuronium and vecuronium. Sugammadex is not metabolized; hence, it seldom causes medication interactions. Additionally, due to their water solubility, sugammadex and the sugammadex-rocuronium complex are removed from the body very quickly.3 The sugammadex-rocuronium combination is stable, making it unlikely that dissociation-related neuromuscular blocking would occur again.
Sugammadex is remarkable for its ability to reverse rocuronium and vecuronium at standard dosages (0.6 mg/kg and 0.1 mg/kg, respectively), as well as deep blockade (e.g., rocuronium at 1.2 mg/kg or given at 1-2 percutaneous transhepatic cholangiography (PTC)), where other reversal medications are unable to restore deep neuromuscular blockade. In contrast to anticholinesterase reversal drugs, sugammadex enables the reversal of any degree of neuromuscular blockade to begin even before spontaneous recovery. This may help to hasten the return of spontaneous respiration. Since the level of neuromuscular blockade (with rocuronium or vecuronium) may be maintained for any amount of time,7 including the profound neuromuscular blockade required in some procedures, sugammadex may be helpful when a surgical procedure ends prematurely.28 Comparing the use of sugammadex with other neuromuscular blockade reversal medications, such as the reversal of cisatracurium with neostigmine plus glycopyrrolate or spontaneous recovery with suxamethonium chloride, demonstrates a clear advantage in terms of the speed of neuromuscular blockade reversal. Sugammadex is effective in certain patient populations, such as pediatric and elderly individuals, as well as those with renal impairment, heart illness, or pulmonary disease. Sugammadex needs more research before it can be considered for use in renal impairment patient since the clearance of the sugammadex-rocuronium complex is greatly delayed in individuals with severe renal impairment.12
Sugammadex effectiveness in restoring common rocuronium-induced neuromuscular blockade, in contrast to neostigmine,29 was unaffected by whether propofol-induced anesthesia was kept up with propofol or sevoflurane. Sugammadex is effective when given to patients who are experiencing deep neuromuscular blockade while under sevoflurane maintenance anesthesia, according to the findings of two studies, even though the impact of various maintenance anesthesia regimens on this efficacy has not been directly compared.30
Suxamethonium chloride treatment is linked to unpredictable, lengthy bouts of neuromuscular blockade (12 to 300-minute relaxation time) in individuals with hereditary abnormalities in the butyrylcholinesterase gene.31 The possible negative consequences of extended neuromuscular blockade might be minimized, and the amount of time patients must spend in the operating room due to incomplete neuromuscular blockade reversal could be decreased if sugammadex could predictably reverse neuromuscular blockade.7 Sugammadex must be administered 15-minutes after the last dose of rocuronium, not based on the degree of neuromuscular blockade, as was the case in some studies. In these patients, however, the time required to reverse rocuronium-induced neuromuscular blockade takes longer than it does in the majority of other patients. In a dosage-finding trial, for instance, five patients who received sugammadex 16 mg/kg (the maximum recommended dose) 15-minutes after rocuronium 1.2 mg/kg experienced a mean TOF 0.9 time of 4.7-minutes as opposed to a range of 0.9 to 16.6-minutes for the other patients. Although the majority of patients (<87%) reached TOF 0.9 within 5-minutes in an observational study by White et al,32 in which 169 evaluable patients received sugammadex 4 mg/kg at least 15-minutes after the last dose of rocuronium (0.6 mg/kg for induction or 0.15 mg/kg for maintenance as needed), 4% of patients reached TOF 0.9 after 5.0 to 22.3-minutes. The twitch response to TOF nerve stimulation in the later study by White et al32 ranged from zero to three twitches at the time of sugammadex administration. Rocuronium is preferred to suxamethonium chloride for usage in rapid sequence intubation despite having a better tolerability profile due to its longer duration of action. Although its usage in this indication has not been studied, the combination of sugammadex and rocuronium may theoretically reduce the duration of action.7 In a “cannot intubate, cannot ventilate” condition, sugammadex may be able to quickly reverse rocuronium; however, because this circumstance cannot be ethically investigated, clinical experience with sugammadex in this setting may assist in addressing its use as a “rescue” reversal drug.7 Neuromuscular monitoring is still necessary despite the extensive and quick reversal because it would help determine the degree of neuromuscular blockade and, consequently, the sugammadex dose needed for reversal. Residual neuromuscular blockade with the recommended use of sugammadex in postoperative care is unlikely.33 The safety of any novel pharmacological drug is of utmost importance. Sugammadex has a typically similar tolerability profile to that of placebo or neostigmine+glycopyrrolate and is well-tolerated in adult surgical patients and in particular patient groups. Among all trial arms, the most frequently reported adverse effects (procedural pain, nausea, and vomiting) are prevalent among people having surgery.34
Sugammadex reduces the likelihood of side effects, including dry mouth, which are frequently linked to neostigmine plus glycopyrrolate or edrophonium with atropine. Sugammadex also does not seem to have any negative cardiovascular consequences, even in people with preexisting cardiac conditions. Importantly, persistent neuromuscular blockade was uncommon when sugammadex was administered at the indicated levels.
The use of sugammadex is subject to several restrictions. Rocuronium or vecuronium should not be administered within 24-hours after the administration of sugammadex if re-paralysis is required (non-steroidal neuromuscular blocking medications are advised in this circumstance). Non-steroidal neuromuscular blocking medications are unaffected by gamma-amidex, and additional medications (such as neostigmine) are necessary to reverse their effects. Sugammadex makes it simple to reverse even deep neuromuscular blockade, which could result in the use of higher doses of neuromuscular blocking medications. Since paralysis can conceal insufficient levels of anesthesia and analgesia, other methods of determining the degree of analgesia and anesthesia may be required.35 Even though an early cost-savings model showed that using sugammadex might save postoperative time in the operating room,36 this was a US-based model, so it might not be applicable in other nations or healthcare systems. Additionally, the cost of purchasing sugammadex was not factored into this model. It would be interesting to do pharmacoeconomic studies using hospital and acquisition expenses from various nations.12
CONCLUSION
Sugammadex is a drug that brings changes in practice of anesthesia and is considered as milestone in clinical anesthesia. It is definitely a boon in the clinical practice of anesthesia in different scenarios with the expedition of NMB reversal. It is a commonly used reversal agent in obese patients coming for bariatric or non-bariatric surgical procedures in developed countries. It has the advantage over neostigmine for quick reversal with minimal side effects. By using sugammadex, unwanted side effects of neostigmine can be prevented. In addition, sugammadex can be used in a wide range of age groups, from pediatrics to geriatrics, and in different clinical settings. Its quick onset of action makes it a novel drug to be used in scenarios like ‘‘Can’t Intubate, Can’t Ventilate”. The future of clinical neuromuscular physiology and pharmacology will see marked development in clinical practice with the use of sugammadex.
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







