OVERVIEW
Vascular complications in renal transplant surgery are extremely uncommon. The complications include renal artery stenosis, renal artery thrombosis, renal vein thrombosis, aneurysm formation, etc. Of this, the renal artery and vein stenosis and thrombosis are more common with an overall incidence of 3-14%, whereas, pseudoaneurysm formation accounts for less than 1% of all vascular complications.
CASE REPORT
A 54-year woman, with a known case of chronic kidney disease (CKD) with hypertension, who was on maintenance hemodialysis of three times per week, underwent a cadaveric donor kidney transplant at our institute on October 13, 2022. The post-operative course was uneventful. She was anuric before the transplant, but had excellent allograft function in the post-operative period, with adequate urine output and normalization of serum creatinine levels.
In the subsequent months, the patient was on regular follow up with good compliance to the prescribed immunosuppresive regimen. Six months after the transplant, on post-operative day (POD) #159, the patient developed fever and malaise. She was treated with antibiotics and the fever subsided over a period of time, only to recur at a later date. The patient was thoroughly evaluated. Her c-reactive protein (CRP) levels were elevated along with her total leucocyte count. Her urine culture was positive for Klebsiella pneumoniae which was sensitive to colistin and tigecycline. She was treated with culture-specific antibiotics and she had normalization of her body temperature for a few days. She subsequently had an exacerbation of her fever with a temperature of 101 F. The patient’s clinical examination was unremarkable, except for being febrile. Her serum chemistries, including creatinine, were also within normal limits.
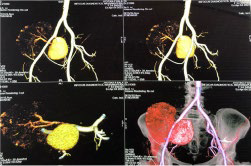
The patient underwent fluoro-D-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) (Figure 1) which revealed a non-FDG avid well-defined, highly enhancing area measuring approximately 50×46 mm in the right renal pelvis adjacent to external iliac vessels—consistent with pseudoaneurysm. The 3D reconstruction (Figure 2) of the CT plates demonstrated an aneurysm of size 50×46 mm arising from the juxta anastomotic site (between the graft renal artery and right internal iliac artery).
Figure 1. FDG-PET/CT Showing an Aneurysm of Size 50×46 mm in Close Vicinity of Iliac Vessels
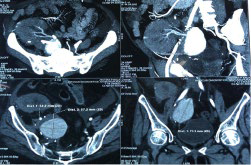
Figure 2. 3D Reconstruction of the FDG-PET/CT Images Revealed an Aneurysm Arising from Juxta Anastomotic Right Internal Iliac Vessel
Renal angiography with digital subtraction angiogram of the right common iliac artery which showed a right proximal internal iliac artery and large pseudoaneurysm. No transplant renal artery was visualized. A 5 F diagnostic Judkins right catheter was placed in the pseudoaneurysm and the angiogram again showed no transplant renal artery. A PROGREAT® microcatheter was placed inside the transplant renal artery and an angiogram was again performed, showing a patent artery and its branches with a slow flow (Figures 3A and 3B).
Figure 3A. Angiogram Showing dye Leaking into the Pseudoaneurysm with Non-Visualization of the Allograft Renal Vessel
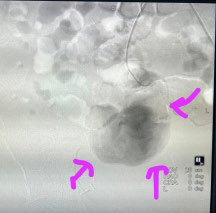
Figure 3B. Angiogram Post Placement of Covered Stent Graft Showing Dye Passing Through the Graft with No Leak into the Pseudoaneurysm and Visualization of the Allograft Renal Vessels
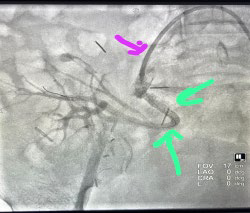
As the patient was hemodynamically stable and possessed favorable anatomy, thus a multidisciplinary decision was made to proceed with endovascular management.
Three Graftmaster (coronary stent graft systems) 16 mm in length and 2.8 mm in diameter were placed to completely cover the area of pseudoaneurysm. Post-procedure right common iliac angiogram showing very good and prompt filling of transplant renal artery through patent stent graft with minimal endoleak. The right external iliac artery flow remained patent post-stent placement.
The post-procedure period was uneventful and color Doppler showed no filling of the aneurysm, and the renal allograft showed normal filling on color mode. There was no visible evidence of arterial stenosis, thrombosis, or perinephric collection. The patient was discharged after 5-days with stable hemodynamic and renal parameters.
DISCUSSION
Vascular complications in post-renal transplant is rare, but result in a high rate of allograft loss. The overall incidence rate of loss with pseudoaneurysm is 6-30%.1 Vascular complications include renal artery stenosis, arterial/venous thrombosis, arteriovenous fistulas, and renal artery pseudoaneurysm, where renal artery stenosis is the most common followed by renal artery thrombosis, renal vein thrombosis and lastly aneurysm formation.2 The aneurysm can be intrarenal or extrarenal (EPSA). Intrarenal aneurysms are most commonly secondary to renal biopsy.
The incidence of extra-renal aneurysms is less than 1%.1 The etiology can be either infectious or non-infectious.3 EPSA can occur at or adjacent to the surgical anastomosis, usually secondary to a mycotic or bacterial infection. Previous literature reviews place the incidence of allograft loss at 56%, concurrence with an infective pathogen at 62%, and mortality at 14%. The mechanism by which infections cause pseudoaneurysm formation is an inflammatory process that invades and compromises the arterial wall’s structural integrity.4
Vascular complications usually occur in the immediate post-operative period but can also present late, like our patient who presented after 6-months. The presentation can be dramatic with possible emergency like sudden cardiovascular collapse secondary to rupture of the aneurysm; or can be a more insidious presentation in the form of abdominal tenderness, pulsatile mass, indolent infection, anemia, and allograft dysfunction. Lastly, there is a chronic, or a late stage, presentation which typically takes the form of diminished allograft function.
In our case, the patient had an insidious presentation, almost 6-months after the transplantation. Her urine culture was positive for Klebsiella pneumoniae; as per the review of the literature, this is the most common bacterial infection associated with EPSA formation.4 She was treated with culture-sensitive antibiotics. The diagnosis can be simplified with a Doppler ultrasound, and more details can be further elucidated by CT angiogram or conventional angiography. Our patient was subjected to a CT angiogram to delineate the anatomical details followed by conventional angiography to decide the precise management approach. The repair can be evoked either by open surgery or lesser invasive modalities, like endovascular management.5 Indications for repair include symptomatic aneurysms, a size larger than 2.5 cm, the presence of infection, and the progressive increase in size that precipitates a life-threatening rupture.6 EPSA smaller than 2 cm can be managed conservatively.
Current literature on this topic quotes allograft nephrectomy as the most definitive treatment option. But with the advancement in endovascular techniques, a greater number of allografts can be salvaged with successful repair of pseudoaneurysm.7 In our case, a multidisciplinary opinion was utilized to develop an endovascular management approach which resulted in the successful exclusion of pseudoaneurysm and well-preservation of allograft renal arterial blood flow.
CONCLUSION
Allograft preservation should be a prime consideration in the management of renal transplant vascular complications. Endovascular management provides a potential definitive cure, with allograft preservation, in properly selected cases. Prevention of urinary tract infection (UTI) post-transplant is of utmost importance as most pseudoaneurysms are of infectious a etiology. Peri-operative bladder irrigation with an aminoglycoside antibiotic solution significantly decreases the overall incidence of UTIs in the first 3-months after kidney transplantation.8
CONSENT
The authors have received written informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









