1. Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (London). 2020; 20: 124-127. doi: 10.7861/clinmed.2019-coron
2. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Eng J Med. 2020; 382: 1708-1720. doi: 10.1056/NEJMoa2002032
3. Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Eng J Med. 2014; 370: 2499-2505. doi: 10.1056/NEJMoa1401505
4. Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020; 395: 565-574. doi: 10.1016/S0140-6736(20)30251-8
5. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020; 579: 265-269. doi: 10.1038/s41586-020-2008-3
6. Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020; 579: 270-273. doi: 10.1038/s41586- 020-2012-7
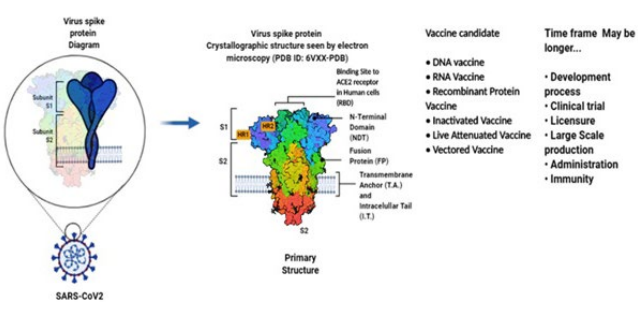
7. Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade – long structural studies of SARS coronavirus. J Virol. 2020; 94: e00127-20. doi: 10.1128/JVI.00127-20
8. Le TT, Andreadakis Z, Kumar A, Roman RG, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020; 19: 305-306. doi: 10.1038/d41573-020-00073-5
9. He Y, Zhou Y, Liu S, Kou Z, Li W, Farzan M, et al. Receptorbinding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004; 324: 773-781. doi: 10.1016/j.bbrc.2004.09.106
10. Le Coupanec A, Desforges M, Meessen-Pinard M, Dube M, Day R, Seidah NG, et al. Cleavage of a neuroinvasive human respiratory virus spike glycoprotein by proproteinconvertases modulates neurovirulence and virus spread within the central nervous system. PLoS Pathogens. 2015; 6: 11,e1005261. doi: 10.1371/journal. ppat.1005261
11. Kirchdoerfer RN, Cottrell CA, Wang N, Pallesen J, Yassine HM, Turner HL, et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016; 531: 118-121. doi: 10.1038/nature17200
12. Walls AC, Tortorici MA, Snijder J, Xiong X, Bosch BJ, Rey FA. et al., Tectonic conformational changes of a coronavirus spike glycoprotein promote membrane fusion. Proceedings of the National Academy Sciences of the United States of America. 2017; 114: 11157-11162. doi: 10.1073/pnas.1708727114
13. Bassi DE, Zhang J, Renner C, Klein-Szanto AJ. Targeting proproteinconvertases in furin-rich lung cancer cells results in decreased in vitro and in vivo growth. Mol Carcinog. 2017; 56: 1182-1188. doi: 10.1002/mc.22550
14. Gupta P. A review: Epidemiology, pathogenesis and prospect in developing vaccines for novel Coronavirus (COVID-19). Indian Journal of Tuberculosis. 2021; 68: 92-98. doi: 10.1016/j.ijtb.2020.09.021
15. Li H, Wang Y, Xu J, Cao B. Potential antiviral therapeutics for 2019 Novel Coronavirus. Zhonghua Jie He He Hu Xi Za Zhi. 2020; 43: E002. doi: 10.3760/cma.j.issn.1001-0939.2020.0002
16. Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronavirus. Front Microbiol. 2020; 11: 298. doi: 10.3389/fmicb.2020.00298
17. Munjal A, Khandia R, Dhama K, Sachan S, Karthik K, Tiwari R. et al. Advances in developing therapies to combat Zika virus: Current knowledge and future perspectives. Front Immunol. 2017; 8: 1469. doi: 10.3389/fmicb.2017.01469
18. Singh RK, Dhama K, Chakraborty S, Tiwari R, Natesan S, Khandia R, et al. Nipah virus: Epidemiology, pathology, immunobiology and advances in diagnosis, vaccine designing and control strategies-a comprehensive review. Veterinary Quaterly. 2019; 39(1): 26-55. doi: 10.1080/01652176.2019.1580827
19. Dhama K, Karthik K, Khandia R, Chakraborty S, Munjal A, Latheef SK, et al. Advances in designing and developing vaccines, drugs, and therapies to counter Ebola virus. Front Immunol. 2018; 9: 1803. doi: 10.3389/fimmu.2018.01803
20. Graham RL, Donaldson EF, Baric RS. A decade after SARS: Strategies for controlling emerging coronaviruses. Nat Rev Microbiol. 2013; 11(12): 836-848. doi: 10.1038/nrmicro3143
21. World Health Organization (WHO). Draft landscape of COVID-19 candidate vaccines. Website. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed February 12, 2021.
22. Callaway E. The race for coronavirus vaccines: A graphical guide. Nature. 2020; 580: 576-577. doi: 10.1038/d41586-020- 01221-y
23. Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020; 581(7807): 215-220. doi: 10.1038/s41586-020-2180-5
24. Li F, Li W, Farzan M, Harrison SC. Structural biology: Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005; 309(5742): 1864-1868. doi: 10.1126/science.1116480
25. Weingartl H, Czub M, Czub S, Neufeld J, Marszal P, Gren J, et al. Immunization with modified vaccinia virus Ankara-based recombinant vaccine against severe acute respiratory syndrome is associated with enhanced hepatitis in ferrets. J Virol. 2004; 78(22): 12672-12676. doi: 10.1128/JVI.78.22.12672-12676.2004
26. Wang C, Li W, Drabek D, Okba NMA, Van Haperen R, Osterhaus ADME, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020; 11: 2251. doi: 10.1038/s41467-020-16256-y
27. Ozkan K. How close are we to a Covid-19 vaccine. Journal of Pure and Applied Microbiology. 2020; 14: 893-902. doi: 10.22207/JPAM.14.SPL1.26
28. Shang W, Yang Y, Rao Y, Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020; 5: 18. doi: 10.1038/s41541-020-0170-0
29. Khatri K, Goyal AK, Gupta PN, Mishra N, Vyas SP. Plasmid DNA loaded chitosan nanoparticles for nasal mucosal immunization against hepatitis B. Int J Pharm. 2008; 354(1-2): 235-241. doi: 10.1016/j.ijpharm.2007.11.027
30. Lee J, Kumar SA, Jhan YY, Bishop CJ. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018; 80: 31-47. doi: 10.1016/j.actbio.2018.08.033
31. Lurie N, Saville M, Hatchett R, Halton J. Developing COVID-19 vaccines at pandemic speed. N Eng J Med. 2020; 382: 1969-1973. doi:10.1056/nejmp2005630
32. Amanat F, Krammer F. SARS-CoV-2 vaccine: Status report. Immunity. 2020; 52: 583-589. doi: 10.1016/j.immuni.2020.03.007
33. Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis. 2013; 208(5); 818-829. doi: 10.1093/infdis/jit236
34. Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. 2015; 19(7): 301ra132. doi: 10.1126/scitranslmed.aac7462
35. Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, et al. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine. 2012; 30: 336-341. doi: 10.1016/j.vaccine.2011.10.085
36. Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, et al. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Neglected Tropical Diseases. 2011; 5: e928. doi: 10.1371/journal. pntd.0000928
37. Mahase E. Covid-19: what do we know so far about a vaccine? BMJ. 2020; 369: m1679. doi: 10.1136/bmj.m1679
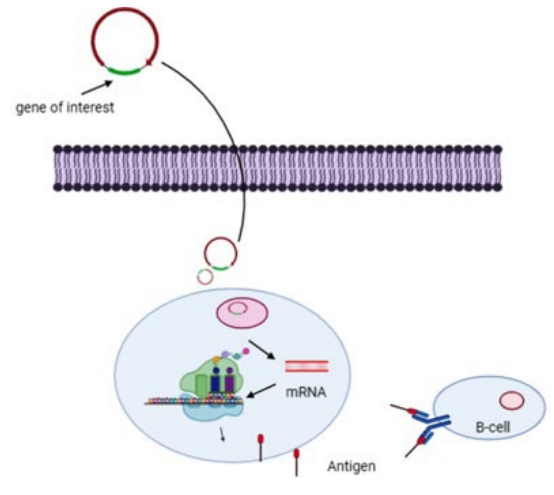
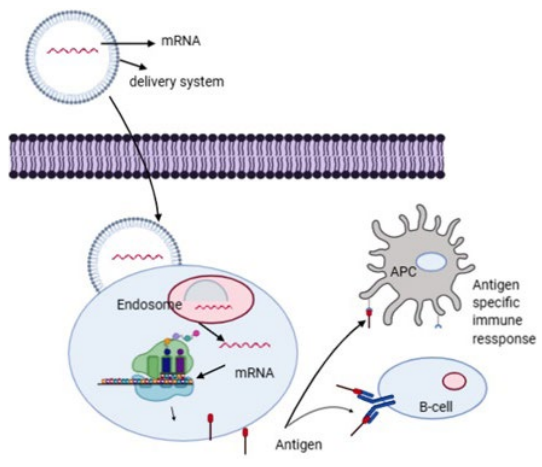
38. Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biology. 2012; 9: 1319-1330. doi: 10.4161/rna.22269
39. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018; 17: 261-279. doi: 10.1038/nrd.2017.243
40. Du L, Zhao G, Chan CCS, Sun S, Chen M, Liu Z. et al. Recombinant receptor- binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009; 393: 144-150. doi: 10.1016/j.virol.2009.07.018
41. Oscherwitz J. The promise and challenge of epitope focused vaccines. Hum Vaccin Immunother. 2016; 12: 2113-2116. doi: 10.1080/21645515.2016.1160977
42. Bachmann MF, Jennings GT. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010; 10: 787-796. doi: 10.1038/nri2868
43. Caivano A, Doria-Rose NA, Buelow B, Sartorius R, Trovato M, D’Apice L, et al. HIV-1 Gag p17 presented as virus-like particles on the E2 scaffold from Geobacillusstearothermophilus induces sustained humoral and cellular immune responses in the absence of IFNg production by CD4+ T cells. Virology. 2010; 407: 296-305. doi: 10.1016/j.virol.2010.08.026
44. Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines. 2020; 8: 153. doi: 10.3390/vaccines8020153
45. Jimenez-Guardeno JM, Regla-Nava JA, Nieto-Torres JL, DeDiego ML, Castano-Rodriguez C, Fernandez-Delgado R, et al. Identification of the mechanisms causing reversion to virulence in an attenuated SARS- CoV for the design of a genetically stable vaccine. PLoS Pathogen. 2015; 11: e1005215. doi: 10.1371/journal.ppat.1005215
46. Plotkin S. History of vaccination. Proc Natl Acad Sci U S A. 2014; 111: 12283-12287. doi: 10.1073/pnas.1400472111
47. Almazan F, DeDiego ML, Sola I, Zuniga S, Nieto-Torres JL, Marquez-Jurado S. Engineering a replication- competent, propagation- defective middle east respiratory syndrome coronavirus as a vaccine candidate. mBio. 2013; 4(5): e00650-13. doi: 10.1128/mBio.00650-13
48. Hou Y, Meulia T, Gao X, Saif LJ, Wang Q. Deletion of both the tyrosine- based endocytosis signal and the endoplasmic reticulum retrieval signal in the cytoplasmic tail of spike protein attenuates porcine epidemic diarrhea virus in pigs. J Virol. 2018; 93: e01758-18. doi: 10.1128/JVI.01758-18
49. Cheng BYH, Ortiz- Riano E, Nogales A, de la Torre JC, Martínez-Sobrido L. Development of live- attenuated arenavirus vaccines based on codon deoptimization of the viral glycoprotein. J Virol. 2015; 89: 3523–3533. doi: 10.1016/j.virol.2016.11.001
50. Mueller S, Stauft CB, Kalkeri R, Koidei F, Kushnir A, Tasker S, et al. A codon- pair deoptimized live attenuated vaccine against respiratory syncytial virus is immunogenic and efficacious in nonhuman primates. Vaccine. 2020; 38: 2943-2948. doi: 10.1016/j.vaccine.2020.02.056
51. Bolles M, Deming D, Long K, Agnihothram S, Whitmore A, Ferris M, et al. A double inactivated severe acute respiratory syndrome coronavirus vaccine provides incomplete protection in mice and induces increased eosinophilic proinflammatory pulmonary response upon challenge. J Virol. 2011; 85: 12201-12215. doi: 10.1128/JVI.06048-11
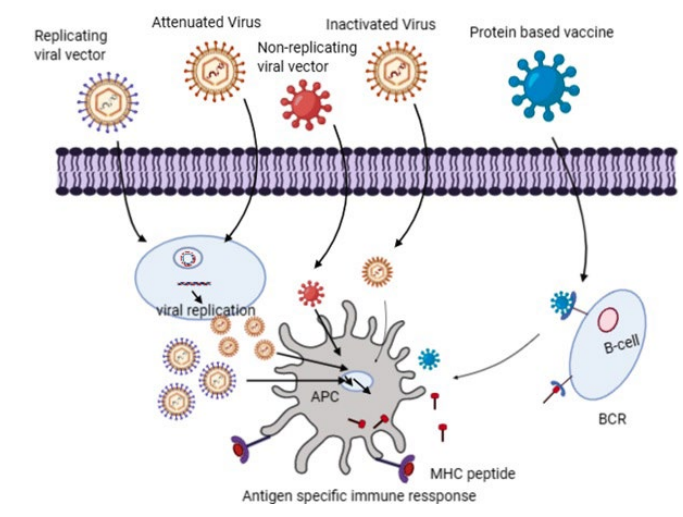
52. Lauring AS, Jones JO, Andino R. Rationalizing the development of live attenuated virus vaccines. Nat Biotechnol. 2010; 28: 573-579. doi: 10.1038/nbt.1635
53. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020; 369: 77-81. doi: 10.1126/science.abc1932
54. Clinical trials.gov. Website. https://clinicaltrials.gov/ct2/show/NCT04456595?term=vaccine&cond=covid-19&draw=2. Accessed May 4, 2021.
55. Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010; 8: 62-73. doi: 10.1038/ nrmicro2240
56. Humphreys IR, Sebastian S. Novel viral vectors in infectious diseases. Immunology. 2018; 153: 1-9. doi: 10.1111/imm.12829
57. Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L. Viral vectors as vaccine platforms: From immunogenicity to impact. Curr Opin Immunol. 2016; 41: 47-54. doi: 10.1016/j. coi.2016.05.014
58. Zhu FC, Li YH, Guan XH, Hou LH, Wang WJ, Li JX, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose- escalation, openlabel, non- randomised, first- in-human trial. Lancet. 2020; 395: 1845-1854. doi: 10.1016/S0140-6736(20)31208-3
59. Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Rammerstorfer SB, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS- CoV-2: A preliminary report of a phase 1/2, single- blind, randomised controlled trial. Lancet. 2020; 396(10249): 467-478. doi: 10.1016/S0140-6736(20)31604-4
60. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020; 20: 339-341. doi: 10.1038/s41577-020-0321-6
61. Tseng CT, Sbrana E, Iwata-Yoshikawa N, Newman PC, Garron T, Atmar RL. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS One. 2012; 7: e35421. doi: 10.1371/journal.pone.0035421