INTRODUCTION
Pituitary adenomas are an adenohypophyseal neoplasm. One meta-analysis of 13 independent studies suggested a prevalence of greater than one in five. This is based on radiological evidence that included both clinically silent and undiagnosed post-mortem cases.1,2 Pituitary adenomas may be categorised: by size where the terms micro- or macro-adenoma are applied; by endocrine function being functioning or non-functioning; by radiological appearance being cystic or non-cystic. Of note, Zhang et al hypothesised that solid pituitary adenomas develop into cystic adenomas as a consequence of either ischaemia or haemorrhage.3 Often, a pituitary lesion is found incidentally when imaging is reviewed for an entirely different indication; these lesions are often termed incidentalomas. An important differential to consider when reviewing pituitary imaging is Rathke’s cleft cyst (RCC). RCC results from failure of proper fusion of the pouch between the adenohypophysis and neurohypophysis, leaving a remnant (cleft) of epithelial cells, known as Rathke’s cleft, which may later, for unknown reason, form a RCC.
Non-functioning cystic pituitary adenomas (cPAs) and RCCs of large enough size may both produce pressure symptoms including headaches, visual disturbance and hypothalamic-pituitary dysfunction. Though distinguishing features may be present on imaging, the mucinous contents of an RCC are of similar signal intensity to a cPA that has undergone haemorrhage or infarction, which is common in larger cysts, making it difficult to differentiate between the two lesions radiologically. Haemorrhage and infarction are less common in RCC despite its population prevalence being up to 33%.4,5,6 Classically, spontaneous resolution of a pituitary cystic lesion is more associated with RCCs than cPAs. However, there are some reports in the literature of pituitary adenomas that spontaneously resolve without associated symptoms of apoplexy.7,8,9 It is thought that spontaneous RCC resolution is perhaps more likely to be under-reported than rare.10,11,12 Transsphenoidal surgery is a definitive management of pituitary lesions, and is required if the presence of the tumour causes symptoms of mass effect or intolerable hormonal imbalance. Furthermore, surgery may prevent recurrence and allows for histological confirmation of diagnosis. However, the natural history of non-functioning pituitary incidentalomas is not fully understood. Despite limited data, it is thought that worsening visual fields, new endocrine dysfunction and apoplexy are rare.13 Though it is useful if classical imaging features are present, it would appear that even in the absence of typical clinical features, asymptomatic small cystic lesions can be conservatively managed as the vast majority of these may remain stable or resolve.
CASE REPORT
The patient is a 67-year-old lady who presented in 2012 due to hemifacial spasm involving the right lower eyelid, for which she subsequently underwent magnetic resonance imaging (MRI) for assessment of her right facial nerve anatomy. A 1 cm pituitary mass was found incidentally present centrally and slightly on left side of midline, having slightly high T1 signal without contrast enhancement and no optic chiasm compression (Figure 1). Vision was normal. The lesion was thought to be cystic with either haemorrhagic or mucinous contents, with possibilities of a cPA and unusual RCC. Further testing revealed a low serum cortisol that responded appropriately to a short synacthen test by rising to 718 nmol/L at one hour. Follow-up scans performed in 2013 and 2014 showed no change. Subsequent MRI in 2015 and 2016 showed progressive reduction in size of the lesion with persistent slightly high T1 signal without obvious contrast enhancement (Figure 2). Further follow-up scan in 2017 showed further significant reduction in size of the lesion, now seen as a tiny eccentric nodule with high T1 signal on superior aspect of left side of pituitary gland (Figure 3). The overall size of pituitary gland appears quite small in Figure 3, with appearance of “empty sella” and prominent cerebrospinal fluid (CSF) space superiorly. In retrospect, the “normal” pituitary tissue in Figures 1 and 2 is also quite less, suggesting that it has been a pre-existing finding and appears more obvious in Figure 3 due to shrinkage of the cyst.
Figure 1. Initial MRI Scan
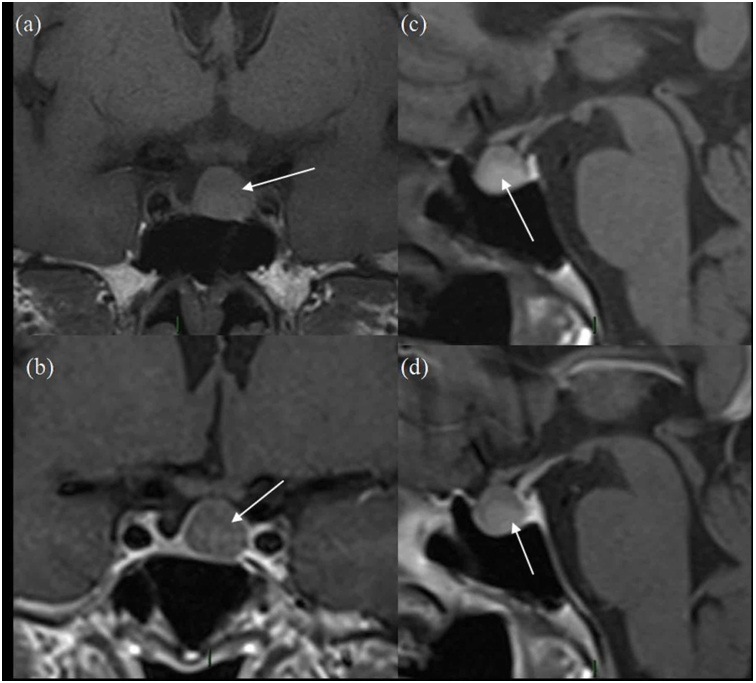
(a) and (b) are pre-contrast and post-contrast coronal images and (c) and (d) are pre-and post-contrast sagittal images. The white arrow represents the pituitary lesion in all sequences, having slightly high T1 signal in (a) and (c) and no enhancement on (b) and (d).
Figure 2. 3-year Follow-up MRI Scans
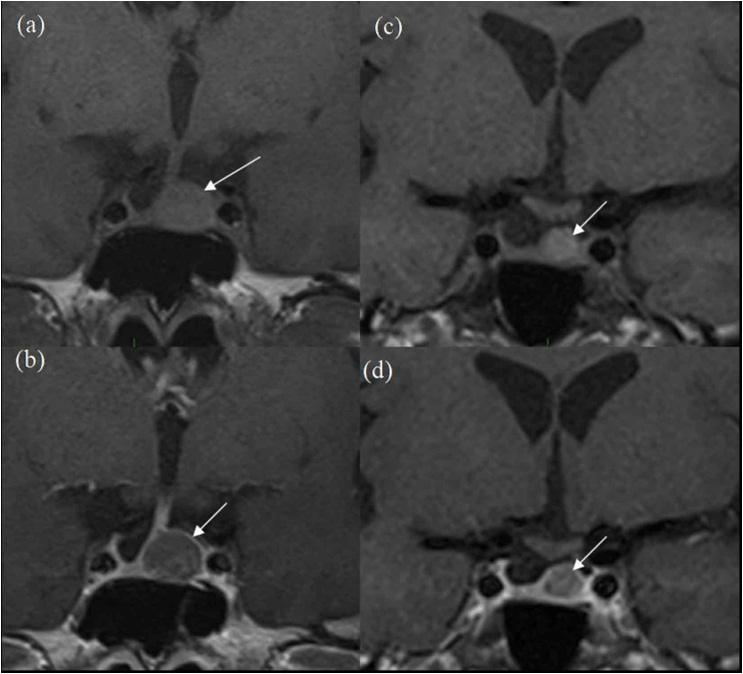
(a) pre-contrast and (b) post-contrast coronal images and more posteriorly (c) pre-contrast and d-post-contrast coronal images. White arrows show progressive reduction in size of the lesion in comparison to Figure 1.
Figure 3. 5-year Follow-up MRI Scan
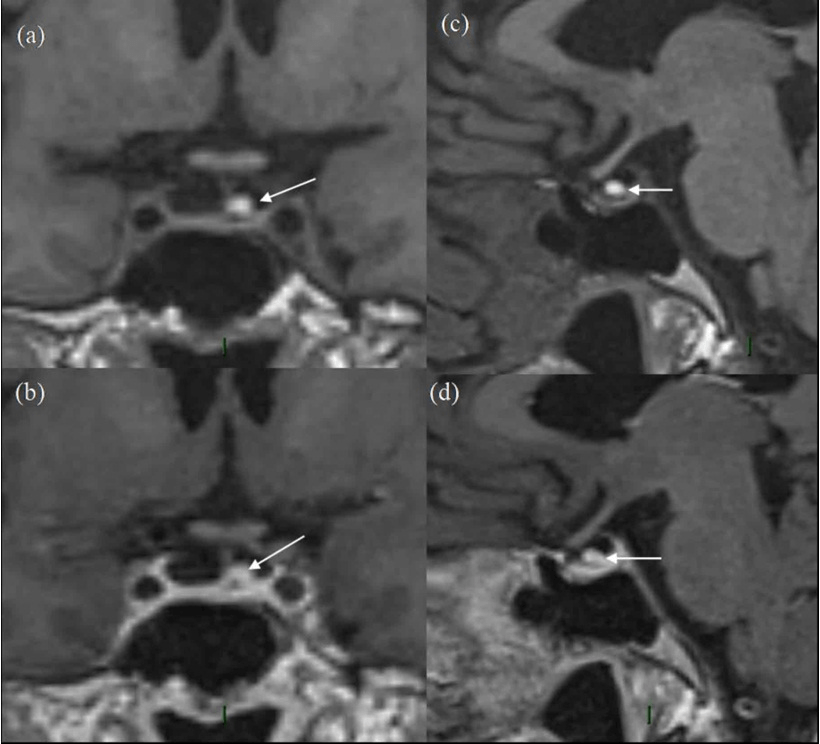
(a) and (b) are pre-contrast and post-contrast coronal images and (c) and (d) are pre-and post-contrast sagittal images. The white arrow represents the residual pituitary lesion in all sequences with significantly reduced size as compared to Figures 1 and 2, having high T1 signal in (a) and (c) and no enhancement on (b) and (d). The overall size of pituitary gland is quite small, with appearance of “empty sella”. In retrospect, the ”normal” pituitary tissue in Figures 1 and 2 is also quite less, suggesting that it has been a pre-existing finding.
Serial MRI, described above, was stopped once the pituitary gland had returned to normal size. Serum urea and electrolytes, full blood count, free T4, TSH, random cortisol, IGF1 and prolactin were normal at all times. Her visual acuity and fields did not deteriorate at any time during her 6-year follow-up of her pituitary cyst, although left cataract surgery was performed 1-year after presentation with right pseudophakia 3-months later without complication; a previous diabetes-induced right branch retinal vein occlusion had been treated with macular grid laser. Visual Acuity was 6/12 right and 6/9 left at time of neuro-endocrinology discharge.
Based on the imaging appearances, RCC and cPA were considered the main differentials. Arachnoid cyst (AC) and craniopharyngioma (CP) are sometimes considered differentials although not in this case due to imaging appearances.
Due to absence of hormonal dysfunction, headache like symptoms or pressure effects, conservative management and annual follow-up MRI scans was performed.
The patient had no clinical signs or symptoms during the entire period of clinical monitoring with spontaneous resolution of her (unrelated) hemifacial spasm, that didn’t recur. Throughout follow-up, she demonstrated no evidence of pituitary dysfunction and developed no headaches or pressure effects. Serial follow-up MRI scans showed the lesion to be stable between 2012 and 2014 and subsequently showed reduction in size, up to 2017, when the lesion was only seen as a tiny residual lesion (Figure 3). No further routine follow-up scans have been performed. The patient has been discharged and is aware that if any of the relevant symptoms develop, the patient can return for further assessment. She is aware that the red flags for surgical intervention, if her cyst were to recur, would be deterioration of visual acuity and/or visual fields. These are being performed annually.
DISCUSSION AND CONCLUSION
Imaging of our asymptomatic patient revealed a small pituitary lesion with usual differentials of cPA and RCC being the focus of this section. RCC tends to be non-enhancing, with rim enhancement often seen in cPA.14,15 In addition, RCC is typically homogenous and found in the midline between the anterior and posterior pituitary lobes, with minimal infundibular deviation as the cyst proper is thought to have soft constituents.16 The presence of a nodule is a feature in 75% of RCC and is almost pathognomonic.17,18,19,20 RCC signal intensity is variable depending on the cyst having mucinous, serous or cerebrospinal fluid (CSF) contents.
cPA is more likely to cause pituitary stalk deviation, with other indicative features being the presence of a fluid level, septation and evidence of intracystic signal change.20 cPA is also more likely to be erosive, with invasion of surrounding sella and extension into the suprasellar space thought to be more characteristic of cPA, or also of CP.21 Calcification is more suggestive of CP, which is often suprasellar in location. RCC and AC rarely extend into the suprasellar space.22 AC tends to present later in life than RCC and CP, the latter two of which may occur in childhood and adolescence. Distinguishing between these pathologies is achievable either intra-operatively, if it is possible to visualise that the AC does not communicate with the intracranial subarachnoid space, or by histopathological examination of the cyst lining, which is generally squamous in CP and cuboidal or columnar in RCC.22 Interestingly, RCC with squamous metaplasia are more likely to recur, and so there is a suggestion in the literature that RCC and CP are more similar than currently thought.21,22,23 Other considerations are dermoid cyst, epidermoid cyst and empty sella. In the current case, the lesion was within the pituitary gland and although the lesion appeared rather central on the initial scans, the last scan showed the lesion to be quite eccentric and probably exophytic, thereby suggesting that cPA is the most likely diagnosis; the lesion also showed no classical features of RCC and CP. The high T1 signal can be assumed to be due to underlying haemorrhage, although it can be argued that some RCCs can show mucinous contents and can show high T1 signal, mimicking blood products; therefore it is difficult to completely exclude an RCC in an unusual location with mucinous contents.
There is no confirmed mechanism attributable to cPA resolution, but there is some suggestion towards that of RCC, which may be applicable to both lesions. Specifically, there exist two hypotheses in the literature regarding spontaneous RCC resolution. The first is that an imbalance between cyst reabsorption and secretion leads to fluctuation in volume of cyst cavity contents, which was first suggested in the context of case reports describing fluctuant visual field defects and MRI imaging demonstrating a change in cyst size but not signal intensity.24 The second is that the cyst capsule ruptures, with or without haemorrhage, thereby facilitating reabsorption. It is rare that RCC present as a mimic of apoplexy, but there is evidence that haemorrhage into the cyst with an associated severe headache does lead to shrinkage.25,26 It is thought that the presence of xanthogranulomatous infiltration, which is a well-recognised histopathological feature of RCC, is indicative of cyst rupture and that a severe, sudden-onset headache as seen in pituitary apoplexy is not necessarily a clinical feature.26 Further case reports ascribe the resolution of cPA to apoplexy.27,28 In the current case, the asymptomatic presentation and clinical course with serial imaging demonstrating high T1 signal would suggest that haemorrhagic rupture in the absence of apoplexy-associated headache can occur followed by spontaneous resolution.
The current case is a reminder that a small asymptomatic cystic pituitary lesion, even in absence of typical diagnostic features, potentially with haemorrhage or proteinaceous contents, can remain stable or spontaneously resolve and can be managed conservatively if there is no risk to vision, and the patient can be counselled on this basis.
CONSENT
The authors have received written informed consent from the patient.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.








