INTRODUCTION
Human papillomaviruses (HPVs) are members of the Papovaridae family of non-enveloped deoxyribonucleic acid (DNA) viruses. They infect the skin or mucosa of vertebrate hosts.1,2 The DNA genome encodes six non-structural proteins (E1, E2, E4, E5, E6 and E7), and two structural capsid proteins (L1 and L2).1 Proteins E1, E2, and E4 participate in viral gene replication, transcription and genome amplification, respectively,1 whereas proteins E5, E6, and E7 are identified as HPV oncoproteins, which initiate HPV infection and the evasion of host immune responses.1 HPVs infect cervical epithelial cells and use the growth and differentiation of these cells to carry out their own life cycle.1 When the HPV genome is transformed from an extra chromosomal state to an integrated phase within the host’s chromosome, the disease progresses from precancerous lesions to high-grade lesions.1 Once the immune system fails to clear persistent HPV infections, there is a high chance that cervical cancer will develop.1
There are currently two prophylactic HPV vaccines: Gardasil and Cervarix, that have been developed and commercialized to the global market.3 They utilize recombinant L1 virus-like particles (VLPs) to induce virus-neutralizing antibodies directed towards conformational epitopes of the L1 capsid protein.4 Both vaccines are able to protect against the most common HPV type, HPV-16, which is responsible for up to 59% of all cervical cancer cases.5 However, these vaccines are only recommended for naïve females, aged from 9 to 26, and not for women already infected with an HPV.6 For this reason, a therapeutic HPV vaccine would be highly advantageous for treatment of the HPV-infected population.
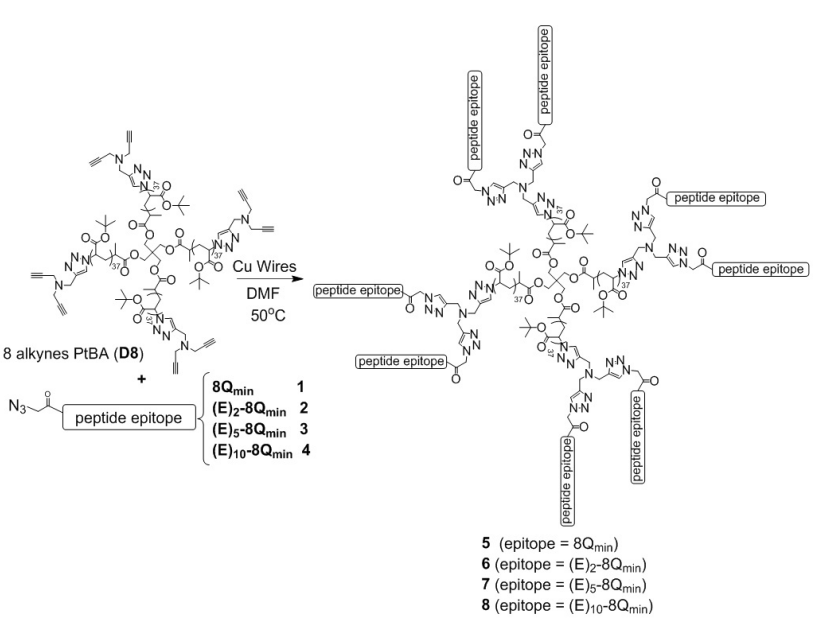
Peptide-based approaches for developing therapeutic vaccines against HPV-associated cancers showed promising results in several early clinical trials.7 In designing synthetic peptide vaccines, the selection of a suitable peptide antigen is a key step. Additionally, cytotoxic T-lymphocyte (CTL-CD8+) epitopes must be included in therapeutic vaccines for cancer treatment.8 Our group showed that 8Qmin peptide, a short fragment of HPV-16 E7 protein (QAEPDRAHYNIVTF; E744-57) bearing CTL and T-helper cell epitopes, can decrease tumor development and eradicate E7-expressing TC-1 tumor cells in mice by activating CTLs.9,10,11,12,13,14 It was shown that 8Qmin was the most relevant peptide epitope to induce desired cellular immunity over the other 8Qmin derivatives.14 However, unprotected peptide epitopes, themselves, are non-immunogenic and easily enzymatically biodegraded in immunized subjects.15 Therefore, an appropriate adjuvant/delivery system is required to boost the immunogenicity of peptide antigens. Self-assembling amphiphilic polymer is one of the most promising delivery systems to carry peptide epitopes.16,17,18,19,20 Liu et al conjugated the peptide epitope 8Qmin to a polyacrylate polymer analogue called 8-arm dendritic polymer (D8, Figure 1) to produce a vaccine construct D8- 8Qmin(5).12 They found that conjugate 5, without the help of an adjuvant, was able to shrink and eradicate HPV-16 E7-positive tumor cells in mice.12 Conjugate 5 self-assembled into 13 μm particles and possessed antitumor potency without the aid of additional adjuvants. Recently, 80% survival rate was observed in model mice after incorporating a lyophilized form of conjugate 5 inside a cationic liposome after single immunization, 7-days post tumor challenge.9 In terms of chemistry, polyacrylates are the polymers of esters of acrylic acid. They are non-toxic and easy to synthesize.21,22 The dendrimer D8 is referred to a polymeric hyperbranched structure: it allows epitope attachment at the periphery of each dendrimer ‘arm’ to create multifunctional biomacromolecules (Figure 1).
A variety of vaccine delivery systems have been designed to form particles that mimic actual infections and the specific sizes of viruses and bacteria.15 However, it has been shown that the immune system reacts most intensively to smaller (less than 200 nm) nanoparticles.15 The most likely explanation for this is that particles smaller than 200 nm can travel to lymph nodes for antigen presentation by themselves,23 whereas larger particles require peripheral dendritic cells (DCs) to transport them to the lymph nodes.14,24 Therefore, we hypothesized that a reduction in the size of conjugate 5 could enhance its potency. To test this hypothesis, 8Qmin was modified with PGA (0, 2, 5 or 10 Glu units, forming 1-4, respectively) to increase its hydrophilicity and, consequently, reduce the size of the self-assembled polymer conjugates 5-8 (Figure 1).
MATERIALS
Protected L-amino acids (Novabiochem, Merck Chemicals, Darmstadt, Germany); rink amide methylbenzhydryl amine (MBHA) resin, dimethylformamide (DMF), dichloromethane (DCM), methanol, diisopropylethylamine (DIPEA), piperidine, trifluoroacetic acid (TFA) (Merck, Hohenbrunn, Germany); copper wires (Aldrich, Steinheim, Germany); hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) (Mimotopes, Melbourne, Australia); high-performance liquid chromatography (HPLC) grade acetonitrile (Lab scan, Bangkok, Thailand); 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU), acetic anhydride, azido acetic acid, acetone, hexane, triisopropylsilane, phosphate-buffered saline (PBS) tablets, sodium hydroxide, β-mercaptoethanol (Sigma-Aldrich, Castle Hill, NSW, Australia); Montanide ISA51 (Seppic, France); dialysis bags (Pierce Snakeskin, MWCO 3K); and Roswell Park Memorial Institute 1640 medium (RPMI 1640), FBS, penicillin-streptomycin-glutamine solution, Trypsin-ethylenediaminetetraacetic acid (EDTA) solution (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were purchased. Alkyne-functionalized 8-arm poly (tert-butyl acrylate) dendrimer with eight alkyne moieties was synthesized, as described previously.16
Equipment and Instruments
Electrospray ionization mass spectrometry (ESI-MS) was carried out using a Perkin-Elmer-SciexAPI3000 instrument and Analyst 1.4 software (Applied Biosystems/MDS Sciex, Toronto, Canada). Analytical reverse-phase high-performance liquid chromatography (RP-HPLC) was performed by an Agilent instrument (Agilent Technologies, Inc., USA). Preparative RP-HPLC was performed on a Shimadzu instrument (Kyoto, Japan). Dynamic light scattering (DLS) was performed using a Malvern Zetasizer Nano Series machine and Zetasizer 6.2 software (Malvern Instruments, United Kingdom). GraphPad Prism 6.00 software was purchased from Graph Pad Software Inc, California, USA.
Synthesis and Characterization of 8Qmin Epitope (E744-57, QAEPDRAHYNIVTF)
The synthesis of 8Qmin was performed according to previously published methods.12,14 8Qmin was synthesized on rink amide MBHA resin (0.79 mmol/g, 0.4 mmol scale, 0.51 g) using solid-phase peptide synthesis (SPPS) at room temperature. Each amino acid coupling cycle contained Fmoc-deprotection with 20% piperidine (5 min; 20 min), a DMF wash (1 min), and double-coupling of preactivated Fmoc-aa (30 min; 60 min). One minute before being added to the resin, Fmoc-aa was activated by dissolving it (0.84 mmol, 4.2 equiv) in a 0.5 M HATU/DMF solution (1.6 mL, 0.08 mmol, 4.0 equiv), then adding DIPEA (0.22 mL, 1.24 mmol, 6.2 equiv). The condition of Fmoc-deprotection for Val, Ile, and Asn was (5 mL of 2% DBU, 5 min, 10 min), while the other amino acids required the condition (5 mL of 20% piperidine, 5 min wash, 20 min wash). Piperidine was replaced by DBU to allow Fmocdeprotection of β-sheet tripeptide unit Val-Ile-Asn, which has a high tendency for aggregation.14 After double-coupling of the first amino acid, the resin was reacted with a fresh acetylation cocktail (0.5 mL acetic anhydride, 0.5 mL DIPEA, 9 mL DMF). The cleavage of 8Qmin peptide was carried out by stirring the resin in TFA (99%)/triisopropylsilane/water (95/2.5/2.5 v/v/v) solution for 4 h. The cleaved peptide was precipitated, filtered, and washed with ice-cold diethyl ether. After lyophilization, crude 8Qmin peptide was obtained as an amorphous powder.
Synthesis and Purification of Peptides 1-4
Synthesis, purification and characterization of peptide 1: 8Qmin azide (peptide 1) was synthesized in the same way as 8Qmin peptide, except the last amino acid was modified with azido acetic acid.12,14 Azidoacetic acid (0.84 mmol, 4.2 equiv) was activated in 0.5 M HATU/DMF solution (1.6 mL, 0.08 mmol, 4.0 equiv.), followed by the addition of DIPEA (146 μL, 0.84 mmol, 4.2 equiv). The peptide-resin was washed with DMF, DCM, and MeOH, then dried in a vacuum desiccator overnight. The peptide was cleaved off of the resin by shaking in TFA/triisopropylsilane/water solution (95/2.5/2.5 v/v/v) for 4 h. The cleavage solution was then evaporated off under reduced pressure. The resin was washed twice with cold diethyl ether/hexane (50/50 v/v) and filtered out with a sintered glass funnel. The precipitate was dissolved in a solution of acetonitrile/water/TFA (50/50/0.1 v/v/v). The peptide dissolved and the resin could be filtered off. The peptide solution was freeze-dried to produce crude peptide as a yellowwhite amorphous powder. The crude peptide was then purified by preparative RP-HPLC on a C-18 column to generate peptide 1. The quality of peptide 1 was analyzed by ESI-MS and analytical RP-HPLC. HPLC analysis (C-18 column): (tR=18.3 min),14 purity >95%. Yield: 37%.ESI-MS: [M+H]+ m/z 1743.85 (calculated as 1743.75), [M+2H]2+ m/z 872.50 (calc. 872.44), [M+3H]3+ m/z 581.95 (calc. 581.96).
Synthesis and purification of peptide 2, 3 and 4: Peptides 2, 3 and 4 were synthesized in the same way as 8Qmin peptide, except the last amino acids were modified with glutamic acid (Glu) using the same procedure for amino acid coupling mentioned above. Peptides 2, 3, and 4 were coupled with two, five and 10 Glu residues, respectively. A coupling cycle of azido acetic acid was then carried out on each sample, as above. ESI-MS and analytical RP-HPLC were used to analyse the peptide products.
Peptide 2, HPLC analysis (C-18 column): (t R=17.5 min), purity >95%. Yield: 27%. ESI-MS: [M+2H]2+ m/z 1001.45 (calc. 1001.56), [M+3H]3+ m/z 667.95 (calc. 668.04).
Peptide 3, HPLC analysis (C-18 column): (t R=17.6 min), purity >95%. Yield: 20%. ESI-MS: [M+2H]2+ m/z 1195.05 (calc. 1195.02), [M+3H]3+m/z 796.95 (calc. 797.01).
Peptide 4, HPLC analysis (C-18 column): (t R=18.1 min), purity >95%. Yield: 15%. ESI-MS: [M+2H]2+ m/z 1518.45 (calc. 1517.13), [M+3H]3+ m/z 1012.15 (calc. 1011.75).
Synthesis and Purification of Vaccine Conjugates 5-8
Copper-catalyzed alkyne-azide cycloaddition (‘click’) reaction: Copper-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reactions were performed similarly to previous studies.10,11,12,14 Peptides 1-4 were individually conjugated to the alkynefunctionalized 8-arm “poly(tert-butyl acrylate)” dendrimer D8 by CuAAC reaction to produce conjugates 5-8 (Figure 1). Freshly washed copper wires were used as a catalyst for each reaction. Copper wires were treated with concentrated sulfuric acid (3 min), washed with distilled water and methanol, then vacuum-dried. Polymer PtBA (D8, 4 mg, 1 equiv.) and peptides 1, 2, 3 and 4 (10 equiv.) were dissolved in DMF (1 mL). The processed copper wires (60 mg) and a clean 0.5 cm stir bar were added into the reaction mixture. The glass flask that contained the reaction mixture was super sealed by rubber cap, and the oxygen was removed by adding nitrogen (30 s). The sealed flask was connected with a nitrogencontaining balloon in an enclosed system. Half of the flask bottom was stationarily dipped into a pre-heated 50 °C oil bath. Both the enclosed system and oil bath were covered by aluminum foil and stirred at 300x for 6-7-hours. The “click” reaction was finalized when the colour of the solution turned light green. The solution (volume: 1 mL) was subsequently filtered via cotton plug into a scintillation vial to remove the copper wires.
Self-assembly: The above filtrates were slowly, individually transferred into a 5 mL syringe. The “click” flask was washed with 0.5 mL DMF, and the solution filtered again. A new scintillation vial containing 4 mL of 1x PBS was prepared, and a clean 1 cm stir bar and 2 μL of 5 M NaOH were added into the vial. The dropping speed of DMF into PBS was set at 0.5 mL/h. The products were allowed to self-assemble into particles by solvent replacement (DMF/PBS). All compounds were then dialyzed against PBS in parallel to remove excess or unreactive peptides and copper salts. Formulated compounds 5-8 were carefully transferred into separate dialysis tubes, which were tightly capped on both ends. Each capped tube was clipped to a float and added into a beaker containing PBS (1 L) and a 5 cm stir bar. The dialysis systems were stirred at 250 X/min and the PBS was renewed three times per day, for three days. After the first three-day dialysis, the solutions were removed for elemental analysis, particle size measurements and in vivo immunizations. All conjugates were observed to form milky suspensions within basic buffer (pH 7.4). In order to complete elemental analysis, 4 mL of each sample was dialyzed against water to remove PBS for an additional three days. The remaining compounds formulated in PBS were used for “DLS and in vivo immunizations.”
Physicochemical Characterization of Vaccine Constructs
Elemental analysis: Samples obtained from the second dialysis were transferred into new scintillation vials, then freeze-dried into solids. In this study, we focused on the changes in the nitrogen/carbon ratio (N/C) on dendrimer D8 before and after peptide conjugation, as previously reported.12 A polymer-peptide conjugate was known to be formed if the conjugate contained a higher N/C ratio than the polymer D8 (N/C=0.017), due to the addition of nitrogenrich peptide onto the polymer.12 The actual conjugation degree of peptide onto the polymer core was calculated using the N/C ratio and an established relationship between conjugation degree and N/C ratio. The actual conjugation degree was then compared to the calculated conjugation degree of polymer fully conjugated with peptides to work out the percentage of conjugation efficiency, as previously reported.12,14
Dynamic light scattering: To perform DLS, 1 mL of each compound formulated in PBS was transferred to a capillary cuvette at 25 °C. Particle sizes were measured in five to eight repeated runs using a Malvern Zetasizer Nano Series instrument with a scattering angle of 173° and correction time of 10 sec per run. The particle sizes (diameter in nm) of each compound were averaged by the Zetasizer 6.2 software.
In vivo Tumor Challenge and Vaccine Treatment
Animals: Thirty female naïve C57 black 6 (C57BL/6) mice (5-7-weeks old) were shipped from the Animal Resources Centre (Perth, Western Australia) to the Biological Research Facility at the Translational Research Institute. All animal protocols used were approved by The University of Queensland Animal Ethics Committee (UQDI/327/13/NHMRC) in accordance with the guidelines of the Australian National Health and Medical Research Council (NHMRC). The mice were separated evenly into six cages (five mice per cage).
Tumor cells and injection: TC-1 cells were generated by transforming murine C57BL/6 lung epithelial cells with HPV16 E6/E7 and ras oncogenes.25 TC-1 cells were cultured and maintained at 37 °C/5% CO2 in RPMI 1640 medium supplemented with 20% FBS, 1×penicillin-streptomycin glutamine solution, and 50 μM β-mercaptoethanol. On the day of the tumor challenge (day 0), adherent TC-1 cells were harvested using 0.25% TrypsinEDTA solution, washed twice with cold PBS, and counted using a hemocytometer. The cells were then resuspended at 5×106 cells/mL in cold PBS. Prior to injection, mice were anesthetized with 4-5% isoflurane at an oxygen flow rate of 0.5 L/min. Each mouse was injected subcutaneously with 5×105 cells suspended in 100 μL PBS into the shaved right flank. On day 7 after tumor implantation, mice were immunized subcutaneously with a single dose of treatment into the shaved left flank. Positive control mice were administered with 30 μg of 8Qmin physically mixed with 50 μL Montanide ISA51 and 50 μL PBS (100 μL total volume). Negative control mice received 100 μL of pure sterile PBS. Test groups received compound 5, 6, 7, or 8 formulated in PBS (100 μL total volume) from the samples achieved after dialysis against PBS.
Tumor measurement: The size of each tumor was measured in two dimensions by electronic digital callipers every Monday, Wednesday and Friday of the experimental period. Tumor volumes were calculated using the formula:
(largest diameter×perpendicular diameter2)
Tumor volume (cm3)= —————————————————————
2
Mice with tumor volumes greater than or equal to 1 cm3 were immediately culled, in-line with ethical guidelines.
RESULTS
Synthesis and Purification of Peptide Epitopes
Peptides 1-4 (Figure 1) were synthesized by stepwise FmocSPPS and individually conjugated to dendrimer D8 to produce four conjugates 5-8, by CuAAC “click” reaction (Figure1). The conjugates were then allowed to self-assemble into particles under aqueous conditions. The conjugation of peptides to the polymer core was confirmed by elemental analysis. The N/C ratio obtained from elemental analysis showed that, on average, 6.7, 6.0, 7.7, and 6.8 peptide molecules were conjugated to the D8 molecule for compounds 5, 6, 7 and 8, respectively. The substitution ratio in compound 7 was the highest at 97%, followed by compounds 8, 5 and 6 with 85%, 84% and 75% respectively (Table 1).
| Table 1. Conjugation Efficiency for Conjugates 5-8 |
|
Theoretical N/C ratio
|
Actual N/C ratio |
Substitution ratio
|
| Conjugate 5 |
0.148
|
0.133 |
84%
|
| Conjugate 6 |
0.153
|
0.128 |
75%
|
| Conjugate 7 |
0.159
|
0.155 |
97%
|
| Conjugate 8 |
0.167
|
0.152 |
85%
|
| D8 |
0.02
|
– |
0%
|
| For each compound, the actual N/C ratio given by elemental analysis was used to calculate the degree of conjugation. The conjugation efficiency was then achieved by comparing this conjugation degree with that of a fully-conjugated polymer D8. A fully-conjugated polymer is known to conjugate with eight peptides over its eight arms. |
Dynamic Light Scattering
DLS spectra (Figure 2) showed that conjugates 5, 6, and 8 formed particles very consistent in size, whereas particles of compound 7 varied in size (Figure 2). Compound 5 formed 3 μm large particles, which were smaller than previously reported (13 μm). This might be related to the higher substitution efficacy (84% vs 76%) achieved in the current study.12 Conjugate particle size decreased proportionally with the number of hydrophilic glutamic acid moieties incorporated into the conjugates.
Figure 2. DLS Analysis of the Average Particle Size (d, nm) of each Conjugate. The DLS Spectra Show the Size Distribution of (a) Conjugate 5, (b) Conjugate 6, (c) Conjugate 7, and (d) Conjugate 8
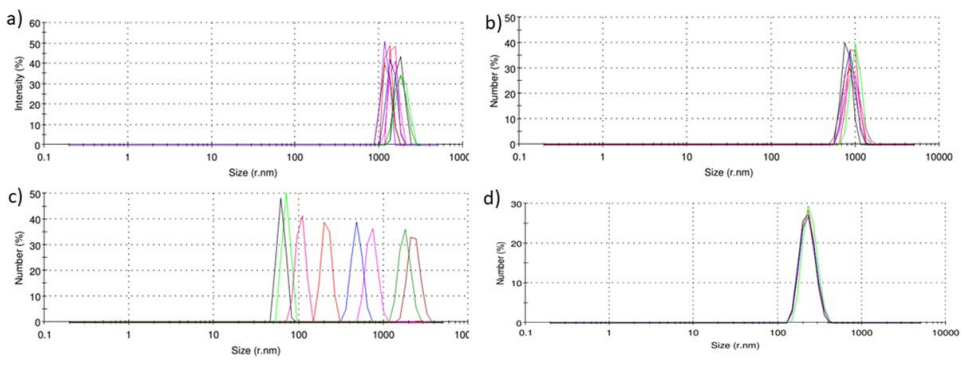
Results for each conjugate were averaged to provide mean conjugate particle sizes. A total of five runs were made for the measurements of compound 5, 6, and 8, while compound 7 was measured by eight runs to further examine the variation in particle size.
In vivo Tumor Challenge and Vaccine Treatment
The in vivo tumor challenge was designed to evaluate the therapeutic efficacy of vaccine candidates 5-8 against established HPV tumors (7-days old) in comparison with positive (8Qmin+ISA51) and negative controls (PBS). Thirty naïve mice allocated to six groups were injected with tumor cells, then received a single treatment dose (100 μL total volume) seven days later. They were palpated and checked for tumor growth every two-to-three days for 44 days.
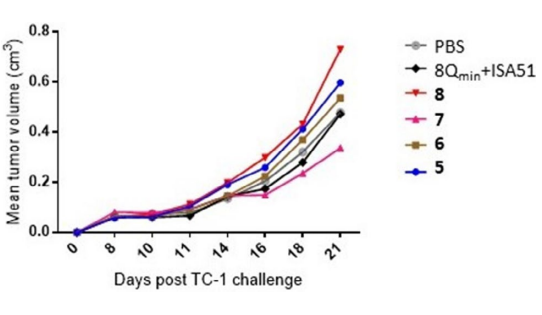
Individual mice were culled when their tumors were equal to or greater than 1 cm3. The average tumor volumes per group were calculated and plotted until the first mouse was culled (Figure 3). All groups showed the same rate of tumor growth until day 10. Tumor growth was slowest in mice immunized with compound 7. On day 21, the average tumor volume of mice immunized with compound 8 was 0.7 cm3 ; while that of compound 7 was 0.4 cm3 . The profile of tumor growth for each individual mouse is shown in Figure 4. It should be noted that no side-effects or allergic responses were reported in any mouse during the in vivo study.
Figure 3. Mean Tumor Volume (Cm3) of Mice Challenged with 5×105 Tc-1 Cells/Mouse (Day 0), Then immunized on Day 7 with Compound 5, 6, 7, or 8, or Positive/Negative Control Solution
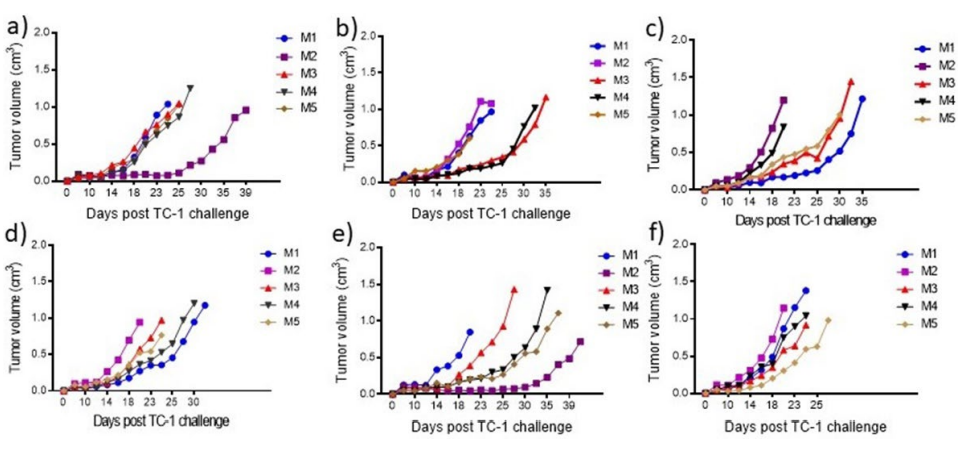
The survival rates of mice in the different test groups directly compared using Kaplan-Meier survival curves (Figure 5). The survival rate of mice immunized with conjugates 5-8 and adjuvanted antigen (8Qmin+ISA51) was not statistically higher than untreated mice (PBS group). At the end of the challenge, only one mouse, treated with compound 7, survived. However, the mouse was not tumor-free and, although the tumor initially shrunk, it regrew rapidly from day 28 (Figure 4e).
Figure 4. Vaccine Immunotherapeutic Effect on the Change in Tumor Volume for each Mouse. The Tumor Volume (cm3
) in Individual Mice Treated with (a) Physical
Mixture of 8Qmin and ISA51, (b) PBS, (c) Conjugate 5, (d) Conjugate 6, (e) Conjugate 7, and (f) Conjugate 8 were Measured from Day 0 to the Day that Mouse
was Culled
DISCUSSION
Oncoproteins E6 and E7 are extensively expressed in all cervical cancer cells. Between these, E7 is more abundant and more capable of immortalizing cells.26 Therefore, the E7 oncoprotein was chosen as an immunogen in this study. To avoid the side-effects associated with the use of whole proteins, the 8Qmin peptide, which contains T-helper cell epitope and CTL epitope, was employed. 8Qmin was conjugated to an 8-arm dendritic polymer delivery platform (D8) to produce self-assembling particles, 5, as previously reported.11-14 In addition, polar poly-(L-glutamic acid) (PGA) linker between the peptide epitope and the polymer was introduced to change the amphiphilic properties of the conjugates, allowing self-assembly into smaller particles.
Four different azide modified epitopes (1-4) were synthesized by Fmoc-SPPS and conjugated individually to a poly(t-butyl acrylate) dendrimer (D8) by CuAAC “click” reaction to obtain conjugates 5-8 (Figure 1). Polyacrylate was chosen as an antigen carrier because it and its analogues have good safety profiles, confirmed pharmaceutical applications,27 and promising adjuvanting capabilities.12,14,28 Dendritic polymer D8 with eight alkyne functional groups was recently found to be a more effective delivery platform for 8Qmin epitope than other polyacrylate analogues.12 The efficacy of 5 (D8 conjugated to 8Qmin) has been reported previously. However, although 5 was able to trigger cellular immune responses that completely eradicated early-stage TC-1 tumors (3-days-old) in mice, its efficacy dropped drastically when used against older tumors (40% survival after 90 days).12 Herein, we modified conjugate 5 with PGA units to increase the hydrophilicity of the peptide epitope.
Figure 1. The Synthesis of Polymer-Peptide Conjugates 5-8 Using Copper Wire-Catalyzed Alkyne-Azide Cycloaddition Reaction
As expected, the addition of Glu moieties into the peptides resulted in changes to the particle sizes of self-assembled conjugates 5-8. The particles formed by compound 6 were twotimes smaller than the particles formed by 5, suggesting that even two additional Glu moieties can influence particle size (Figure 2). Compound 8, which contained the highest number of Glu units, produced the smallest particles (460 nm); therefore, the number of Glu units showed direct correlation with the size of particles produced. Unexpectedly, compound 7 formed particles in a wider size range than the other conjugates. The inconsistent self-assembly properties of conjugate 7 could have been driven by electrostatic interaction and hydrogen bonding between different particles facilitated by hydrophilic segments of the peptides, resulting in multiple conjugates aggregating in different self-assembly patterns.
The therapeutic effects of the developed vaccines were evaluated by the ability of conjugates 5-8 to eradicate tumors in female C57BL/6 mice. For this study, a transplantable tumor model was used as a substitute for natural HPV-induced tumors. In this model, murine epithelial TC-1 cells were co-transformed with HPV-16 E6, E7, and ras oncoprogenes, and propagated in inbred mice.25 The tumors formed by these cells have been reported to be susceptible to peptide-based immunogens of therapeutic vaccine constructs in various studies.29,30,31,32 As a follow-up to our previous studies,12,14 we established this preclinical model to test for HPV-specific immune responses following tumor challenge.12 Mice treated with the most polydisperse conjugate 7, showed the slowest tumor progression and the best survival rate (Figure 5). Interestingly, compound 5 was less effective at triggering antitumor immune responses than reported previously. Hussein et al found that mice vaccinated with conjugate 5 had significantly reduced tumor volumes and improved survival rates (4/10) compared to positive (2/10) and negative control mice (0/10).11,12 The weaker than anticipated effectiveness of conjugate 5 in the present study could be explained by several theories: (1) tumor growth rate inconsistency between studies; even comparatively tiny variability in the amount of inoculated tumor cells can have a major impact on tumor development;33,34 (2) variability in the sensitivity of C57BL/6 mice to TC-1 tumors;35 and (3) variation (even if minor) in the properties of conjugate 5 between distinct synthetic lots.9
Figure 5. Survival Rates of Mice. The Survival Rate of each Group of Mice was Recorded from Day 0 to Day 44, when the Last Mouse was Culled
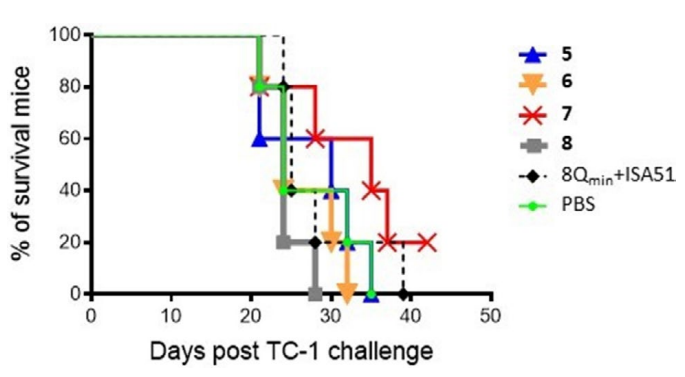
The survival rate of each group was plotted on a Kaplan-Meier survival curve, and was compared to that of the positive control (8Qmin+ISA51). The difference in survival rates between groups was not statistically significant (p>0.05).
Unfortunately, the reduction of particle size in the polymer-antigen conjugates from 13,000 nm (5) to 430 nm (8) did not improve antitumor efficacy. This highlights that further optimization of vaccine structures and dosing is required to target HPV-associated lesions. The number of immunosuppressive cells at the time of vaccine administration should be reduced to enhance CTL responses. For example, in vivo depletion or inactivation of Tregs has been proven to allow a strong intratumoral invasion of CTLs and complete eradication of HPV-associated tumors in mice36,37,38,39 and humans.40,41
CONCLUSION
Four different peptides, 1-4, containing 8Qmin and PGA of different lengths, were successfully synthesized with high-yield and high-purity. These peptides were conjugated to the polymer D8 to generate four different vaccine candidates, 5-8, which were then self-assembled into particles. The size of the formed particles was controlled by PGA length. Vaccine compounds 5-8 were administered to tumor-bearing C57BL/6 mice, and compound 7 was found to be the most potent vaccine candidate with a 20% survival rate. Results suggest that the size of the nanoparticles did not influence potency, contrasting a variety of previous reports that showed that “smaller is better”. We suggest that future vaccination strategies include additional boost vaccinations, as well as immunological monitoring of the target tumor microenvironment following initial vaccination to boost T-cell immune responses.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










