INTRODUCTION
The strong upward rise in cancer-drug prices and spending has provoked concern on drug wastage and called for a search of saving measures. Cancer is one of the top leading causes of death worldwide and considered as the 2nd most common cause of death in the United States. About 589,430 American are expected to die of cancer in 2015, that’s about 1,620 people per day. The expected number of newly diagnosed cases of cancer in American population in the year of 2015 is around 1,658,370 cases. The Agency for Healthcare Research and Quality (AHRQ) estimated that the direct medical costs for cancer in the US in 2011 was $88.7 billion.1 In 1990, costs of cancer care were estimated to be 27 billion dollars, rising to 90 billion dollars in 2008, and expected to be around 157 billion dollars by 2020, which is almost a 600% increase over the past 3 decades.2,3
With the rising costs of cancer care and number of newly introduced expensive therapies, cost containment strategies are important. Drug wastage as a consequence of unused or partially used vials can be costly, while dose rounding of both chemotherapeutic and biologic anti-cancer agents to the nearest vial size has resulted in substantial cost savings.4 It entails calculating the dose of drug based on the patient’s body surface area (BSA) and the approved dosing regimen. The calculated dose is then rounded within a percentage limit (10% is the most commonly used in the literature) to the nearest vial size. This approach have been found to reduce drug wastage and to be more cost effective, since it permits the complete use of the content of any given vial.5
In this study, we investigated the potential cost savings associated with dose rounding of standard chemotherapy regimens to the nearest vial size.
METHOD
This retrospective quantitative study was conducted at King Abdulaziz Medical City (KAMC), Riyadh, Saudi Arabia oncology satellite pharmacy. Preliminary data were collected from chemotherapy pharmacy satellite work sheet for the period of the study starting March 1st to March 31st, 2015. All adult patients aging 14 years and older with a diagnosis of cancer for which they have received anti-cancer agents during the study period were included (N=305). Doses of anti-cancer drugs were calculated based upon the patient’s BSA and the Food and Drug Administration (FDA) approved recommended dosing regimen. The exact total dose was then theoretically rounded to the nearest vial size. The maximum dose rounding allowed was 10% for palliative intent treatment and 5% for curative intent treatment. The rest of the demographic data were completed from the computerized health information system (Quadra-Med) using a data collection sheet. The new theoretically rounded doses costs were analyzed to estimate the potential cost savings in comparison to the actually dispensed doses considering the maximum allowed rounding to the nearest vial size (5% for curative intent and 10% for palliative intent). The potential effect on cost was calculated in Saudi Riyals (SR) for both the actual doses dispensed calculated and rounded doses. Drug wastage was defined and calculated as the amount of drug (number and size of vials) that may have been saved if the rounding to nearest vial size was implemented. Data collected included drug name, ordered dose, rounded dose, number of product vials wasted, and drug direct cost. In our study, all Institutional Review Board (IRB) procedures were followed.
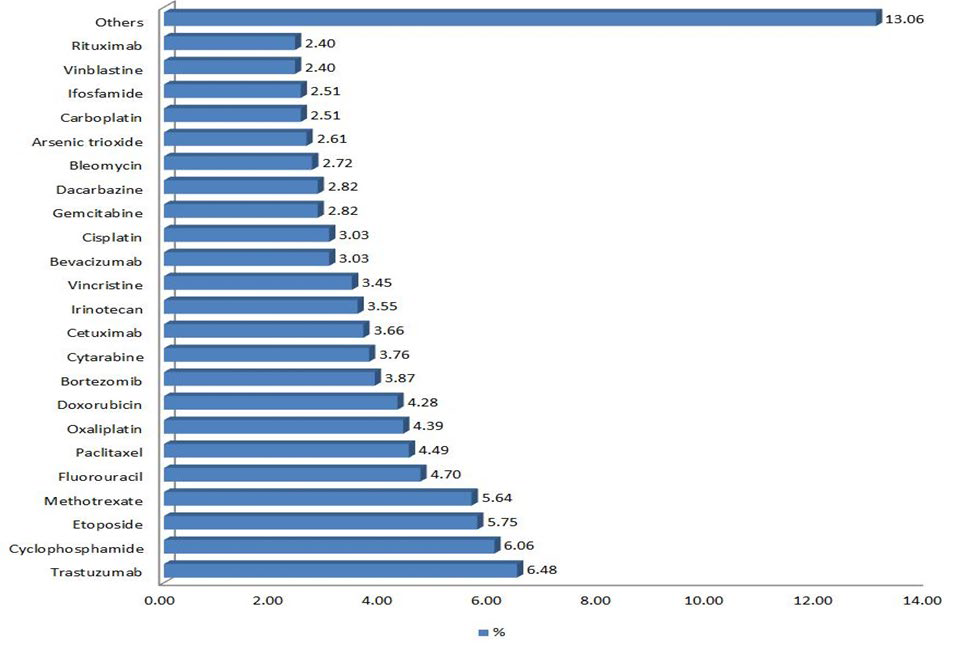
Figure 1: Anti-cancer agents with the most drug wastages saved when rounding to the nearest vial size.
RESULTS
A total of 305 patients received 973 doses of intravenous chemotherapy and anti-cancer biological agents during March 2015. Among these 973 doses, 352 doses could be rounded to the nearest vial size with a 10% dose deviation for palliative intent and 5% for curative intent. The projected savings for one month was calculated to be SR 14,720.95 for 5% rounding limit and SR 53,157.50 for 10% rounding which accounts to an estimate of SR 814,514.40 of drug wastages per year (Table 1). Anti-cancer drugs with the most drug wastage were trastuzumab, cyclophosphamide, etoposide, methotrexate, fluorouracil, paclitaxel, oxaliplatin, doxorubicin, bortezomib, and cytarabine consecutively (Figure 1). The highest costs savings when rounding the doses to the nearest vial size up to 5% for curative intent were associated with rituximab, followed by fludarabine and paclitaxel. Bevacizumab, cetuximab, and carfilzomib have resulted in the most cost savings when the rounding limit was set to 10% for palliative intent. In our study we found out that around 38.69% (N=118) of the patients were treated with a curative intent while 61.31% (N=187) of the patients received palliative chemotherapy (Figure 2). The most common type of cancer was breast cancer which has been seen in about 20% (N=61) of the cases (Figure 3). Female patients comprised 59.67% (N=182) of the patients and those who aged between 51-60 years were the most age group to receive chemotherapy agents 21.64% (N=66) (Figures 4 and 5).
Figure 2: Treatment intent.
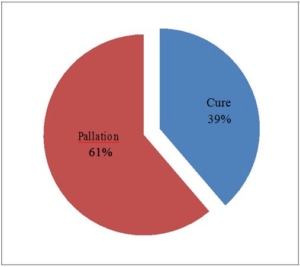
Figure 3: Diagnosis.
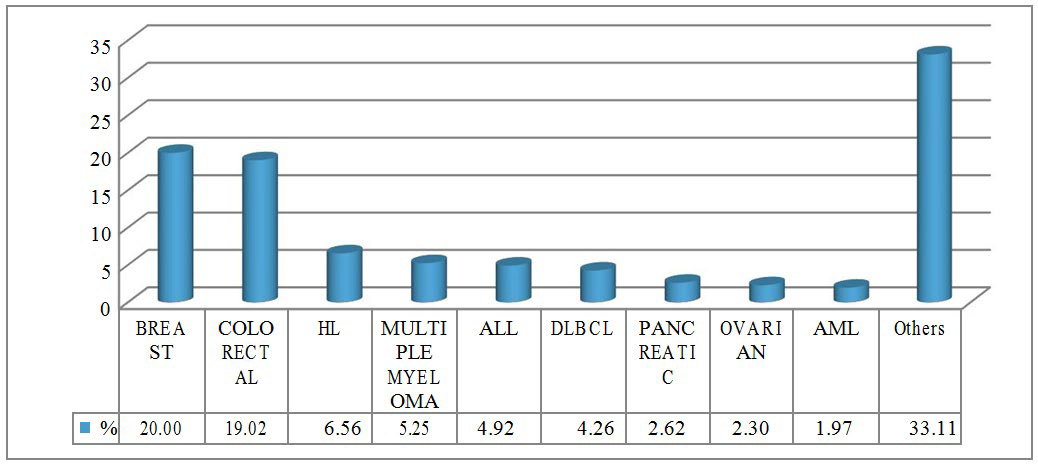
Figure 4: Gender
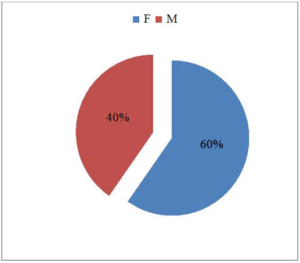
Figure 5: Age group
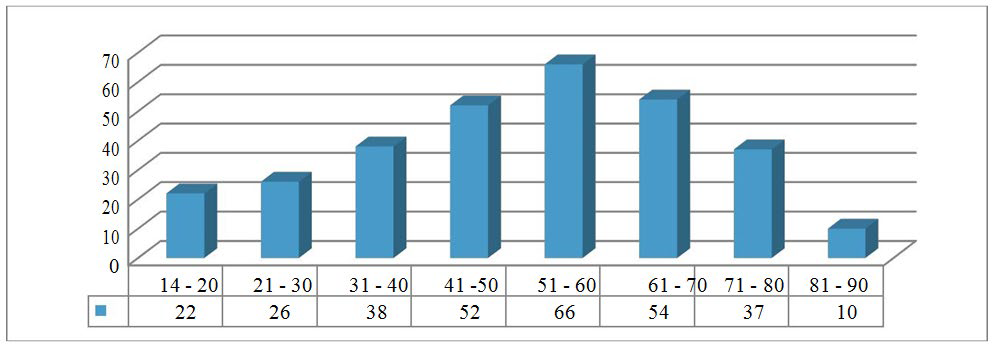
Table 1: Cost saving from rounding up to 5% for curative intent, and up to 10% for palliation intent.
|
Intent of treatment (Cure of Pallation)
|
Medication |
Sum of # of Vials saved 2 |
Sum of cost saved (1 mo) SR. |
Sum of cost saved (12 mo) SR. |
|
Cure (Rounding up to 5%)
|
Cisplatin
|
3 |
85.05 |
1,020.60
|
|
Cyclophosphamide
|
14 |
653.80 |
7,845.60
|
|
Cytarabine
|
2 |
299.20 |
3,590.40
|
|
Etoposide
|
2 |
586.20 |
7,034.40
|
|
Fludarabine
|
3 |
4,889.25 |
58,671.00
|
|
Methotrexate
|
1 |
21.65 |
259.80
|
|
Paclitaxel
|
4 |
2,309.60 |
27,715.20
|
|
Rituximab
|
2 |
5,876.20 |
70,514.40
|
|
Toatal
|
31 |
14,720.95 |
176,651.40
|
|
Pallation (Rounding up to 10%)
|
Bevacizumab
|
10 |
15,505.00 |
186,060.00
|
|
Carboplatin
|
3 |
583.50 |
7,002.00
|
|
Carfilzomib
|
1 |
8,250.00 |
99,000.00
|
|
Cetuximab
|
9 |
11,844.00 |
142,128.00
|
|
Cisplatin
|
2 |
85.05 |
1,020.60
|
|
Cyclophosphamide
|
8 |
373.60 |
4,483.20
|
|
Cytarabine
|
3 |
448.80 |
5,385.60
|
|
Docetaxel
|
2 |
1,430.00 |
17,160.00
|
|
Fluorouracil
|
2 |
50.50 |
606.00
|
|
Gemcitabine
|
14 |
1,757.00 |
21,084.00
|
|
Irinotecan
|
4 |
2,086.80 |
25,041.60
|
|
Oxaliplatin
|
12 |
2,962.20 |
35,546.40 |
|
Paclitaxel
|
8
|
4,619.20
|
55,430.40
|
|
Rituximab
|
1 |
2,938.10 |
35,257.20
|
|
Vinblastine
|
1 |
223.75 |
2,685.00
|
|
Toatal
|
80 |
53,157.50 |
637,890.00
|
| Grand Toatal |
67,878.45 (18,101 USD) |
814,541.40 (217,211 USD)
|
DISCUSSION
A significant and progressive cost rising in medical oncology due to the incorporation of novel and highly expensive drugs into clinical practice have been seen in the past years. Intravenous cancer agents are typically supplied in fixed drug amount vials, allowing the use of partial vials for a given patient. Some drugs, and the majority of monoclonal antibodies, are packaged preservative free, allowing only for single time uses with a short expiration. When expensive new drugs, such as monoclonal antibodies or other biologics, are made preservative-free and used infrequently, unused partial vials that have expired can account to a large amount of drug wastage. Drug wastage is defined as the consequence of an inappropriate disposal of unused or partially used drug vials or syringes.6 Dose rounding is an option which might be used in oncology settings to cut down extra costs due to drug wastages. Literature suggests that dose rounding of chemotherapy and biologic drugs to an amount of up to 10% of the ordered dose is a significant cost-containment in an era of rising health care costs without adverse effects or a negative impact on the treatment outcomes.5
For instance, rituximab which is available as 400 mg and 100 mg vials, if the calculated dose is 720 mg, it could be rounded to 700 mg by 2.8% dose deviation. In such a case, one 100 mg vial will be saved and limit a potential distinct economic loss. Brenda et al5 found that dose rounding could reduce drug wastage for up to 42% and resulted in a potential cost savings of $24,434 for 3-month interval. In another study done by Nagwa Ibrahim, 7 dose rounding of anti-cancer agents may save around $192,800 per year. Our study reveals that the projected annual saving was calculated to be SR 176,651.40 for 5% rounding limit and SR 637,890.00 for 10% rounding which will account to an estimate of SR 814,514.40 ($217,204) of drug wastage per year. Trastuzumab, and cyclophosphamide were the most anticancers associated with drug wastage. Rituximab has resulted in the highest costs of drug wastage when rounding the doses to the nearest vial size up to 5 % for curative intent. While bevacizumab, and cetuximab have resulted in the most cost savings when rounding to 10% for palliative treatment. Female patients were more to receive chemotherapy than males in our population and this could be correlated with the fact that the most common type of cancer in our study to receive chemotherapy was breast cancer. Around 61.31% of chemotherapy orders were for palliative intent treatment which could allow more space for rounding up to the limit of 10% to the nearest vial size and contribute to more potential cost savings. The most age group to receive chemotherapy in our study was those who aged 51-60 years 21.64% (N=66) followed by those who aged between 61-70 years old 17.70 % (N=54).
In our study, dose rounding of anticancer agents to an amount within 10% of the ordered dose has resulted in cost savings through reduction of drug wastage in our institution and is consistent with the findings of other studies. As it is a one month retrospective study, a prospective study with a longer duration and a larger population might help in getting a more accurate projection of drug wastage and cost savings with dose rounding strategy to the nearest vial size. Clinical outcomes of dose rounding could be another future research focus as well. Doserounding interventions by a pharmacist are feasible and an automatic dose-rounding protocol is desired to assist in reining the rising cancer drugs expenditure.
CONCLUSION
Dose rounding of the ordered dose to an amount within 5% for curative intent and 10% for palliative intent to the nearest vial size has resulted in a valuable cost savings through reduction of drug wastage.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










