INTRODUCTION
Thrombelastography (TEG) was developed during World War II by Professor H. Hartert in Heidelberg.1 Following a quite broad application in the 50’s and 60’s, the interest in TEG decreased in the 70’s. In the 80s came a renaissance of TEG, especially in the United States, because of its application in anesthesia for the management of acute bleeding. The ROTEM® system is an enhancement of thrombelastography and was developed during 1995-1997 in Munich.2 The instrument includes four measurement channels for simultaneous determinations, an integrated computer for automatic analysis, and an electronic pipette for interactive test operation.
Vasodilatation effects of anesthetics may induce hypotension, which may reduce hemorrhage, but they may also cause microvascular hemorrhage, even if systolic cardiac output is decreased. “Thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®)” give a global picture of how clots form, stay together, and break up in living organisms.3 ROTEM®, a point-of-care viscoelastic technique, can be used in a variety of medical settings to measure the viscoelastic properties of blood cells.4 ROTEM® makes use of a variety of stimulants and regulators to examine the link between polymerizing fibrin and blood cells, producing thrombin, and potential clot breakdown. This article’s objective is to demonstrate the significance of ROTEM’s usefulness in anesthesia practice. The term “point-of-care viscoelastic method” can be used to describe ROTEM® anesthesia, also known as ROTEM®. Anesthetists can typically estimate “viscoelastic profiles” of the entire blood in a variety of clinical settings. Consequently, this article will focus on the use of ROTEM® in anesthesia where substantial blood loss is expected, such as in major cardiovascular surgery, liver transplantation, trauma, and post-partum hemorrhage (PPH).5
PRINCIPLES AND COMPONENTS OF ROTEM®
Hartert first described TEG in 1948.1 ROTEM® is based on this principle. ROTEM® employs a pin suspended in a blood sample-containing cuvette that is rotated back and forth by a motor. There is a one-millimeter gap between the pin and the cuvette, which is filled by blood or a blood clot. The pin is attached to a torsion wire that measures the blood clot’s resistance to rotation.6 As long as blood is liquid, the pin can move freely. When blood clots form, they restrict the rotational movement of the pin, so the rotation of the pin is inversely proportional to the clot’s firmness. The rotational movement generates a viscoelastic signal, which a computer records and displays as a graphical trace. The trace provides several parameters that describe the various stages of coagulation and exhibit changes in clot elasticity over time.
ROTEM® CONSISTS OF FOUR MAIN COMPONENTS
The Analyzer
This is the device that moves the pin in a rotating motion and records the viscoelastic signal. There are two types of analyzers: the ROTEM® delta and the ROTEM® sigma. The ROTEM® delta is a manually operated device that requires the user to pipette the blood sample and the reagents into the cuvette and insert it into one of the four channels of the analyzer. The ROTEM® Sigma is an automated device that requires the user to load the blood sample into a cartridge and insert it into one of the eight channels of the analyzer. Then the instrument performs the analysis while automatically adding the reagents.6
The Cuvette
It is a disposable plastic container that holds 300 µl of blood sample and 20 µl of reagent. A pin is attached to the cuvette’s lid and submerged in the blood sample. Additionally, the cuvette has an optical sensor that measures the blood sample’s temperature and sets it to 37 °C.
The Reagent
It is a liquid substance that either activates or inhibits a specific coagulation pathway or component. Depending on the clinical question and the assay, different types of reagents can be used with ROTEM®. Tissue factor (TF), which activates the extrinsic pathway; ellagic acid (EL), which activates the intrinsic pathway; cytochalasin D (CyD), which inhibits platelet function; heparinase (HEP) which neutralizes heparin; and aprotinin (AP), which inhibits fibrinolysis are the most frequently used reagents.7
The Software
This is a computer program that manages the analyzer, shows the graphical trace, computes the parameters, and offers interpretation and suggestions based on predefined algorithms (Figures 1 and 2).
Figure 1. ROTEM Illustration18
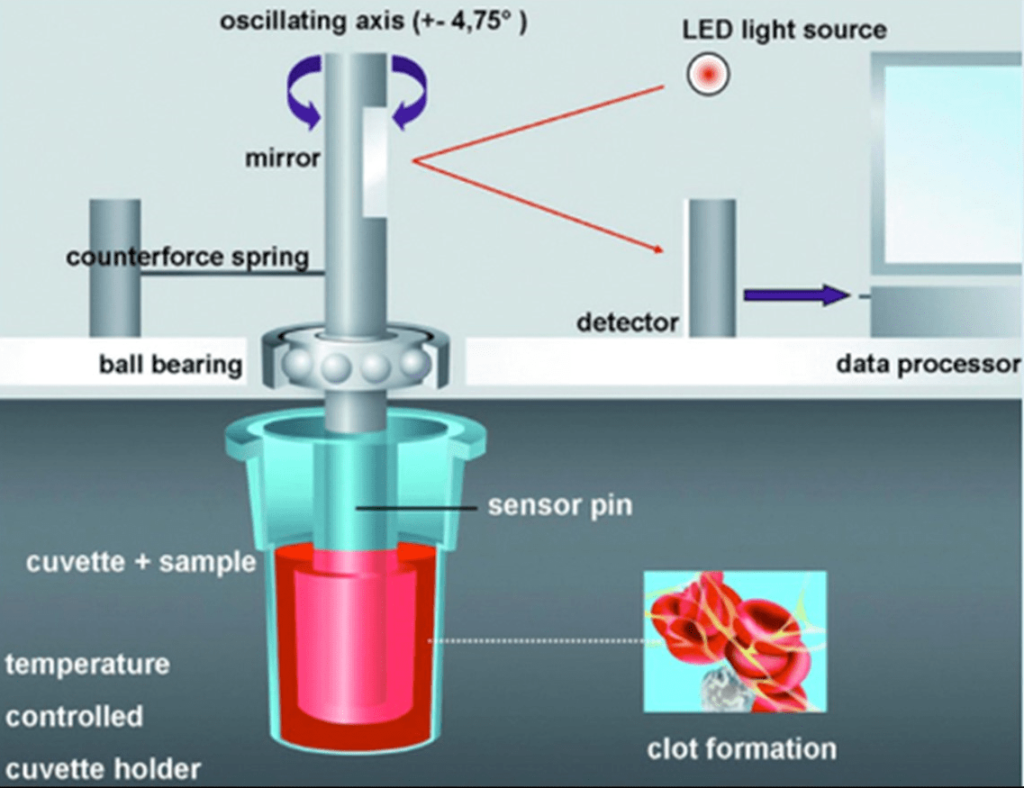
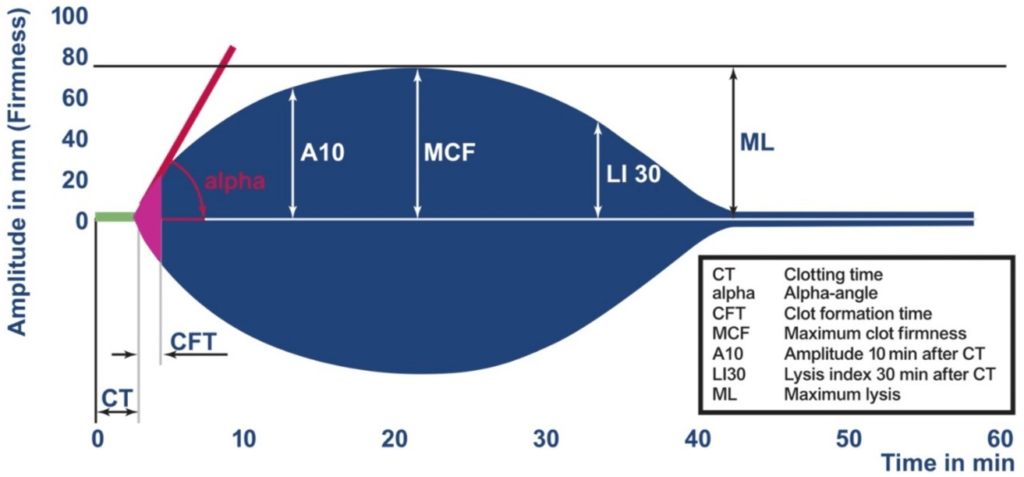
Figure 2. ROTEM Thromboelastometry Parameters and Scaling18
TYPES AND INTERPRETATION OF ROTEM® ASSAYS
Various ROTEM® assays can be carried out depending on the clinical question and the reagents that are available (Figure 3). Among the most typical ones are:
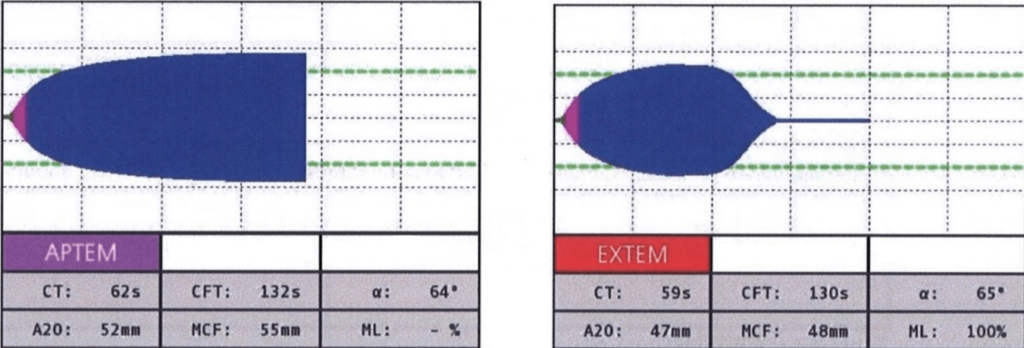
EXTEM: This assay uses TF (tissue factor- tissue thromboplastin) as the reagent to activate the extrinsic pathway. It demonstrates the contribution of factors II, V, VII, and X, along with platelets and fibrinogen, to clot formation and lysis. This involves the beginning of clot formation within 70 seconds, and clot formation time can be evaluated within 10-minutes. It is helpful for determining global coagulation function, detecting factor deficiencies or inhibitors, monitoring heparin reversal, and guiding transfusion therapy (Figure 3).8
Figure 3. Normal EXTEM18
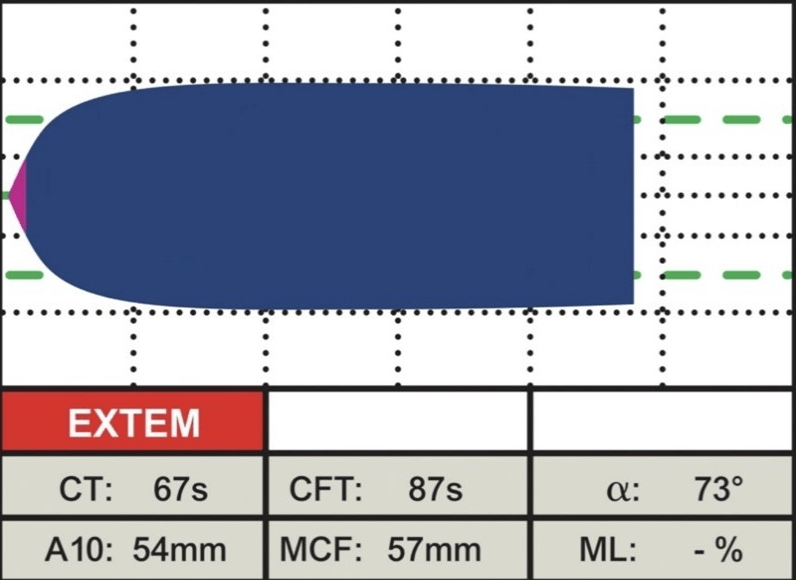
INTEM: This assay uses EL as the reagent to activate the intrinsic pathway. In this instance, contact factors activate coagulation, demonstrating the contribution of factors II, V, VIII, IX, X, XI, XII, heparin, and fibrinogen to clot formation and lysis. It is useful to detect contact factor deficiencies or inhibitors, monitor heparin therapy, and diagnose heparin-induced thrombocytopenia (Figure 4).
Figure 4. Normal INTEM18
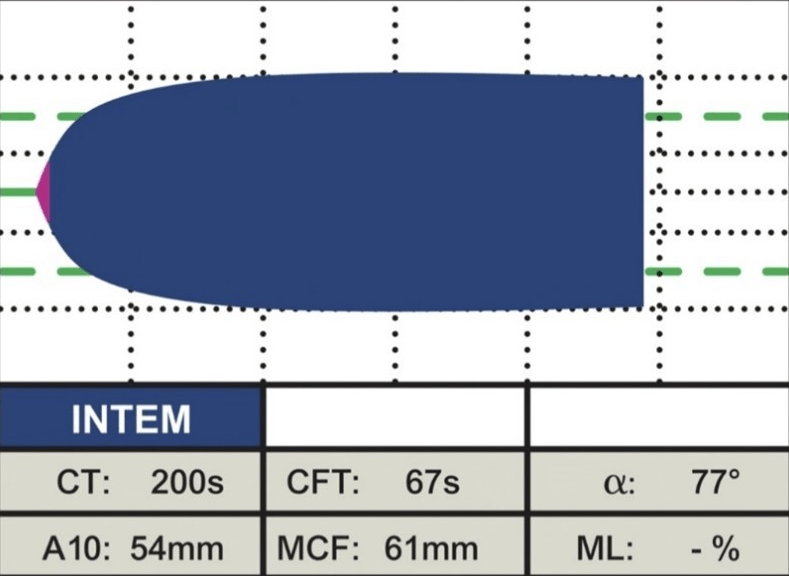
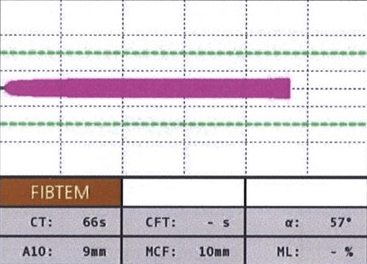
FIBTEM: This assay uses TF and CyD as the reagents to activate the extrinsic pathway (EXTEM) and inhibit platelet function. It reflects only the contribution of fibrinogen to clot formation and lysis. It is useful to assess fibrinogen level and function, detect fibrinogen deficiency or dysfunction, monitor fibrinogen replacement therapy, and diagnose hyperfibrinolysis (Figure 5).
Figure 5. Normal FIBTEM18
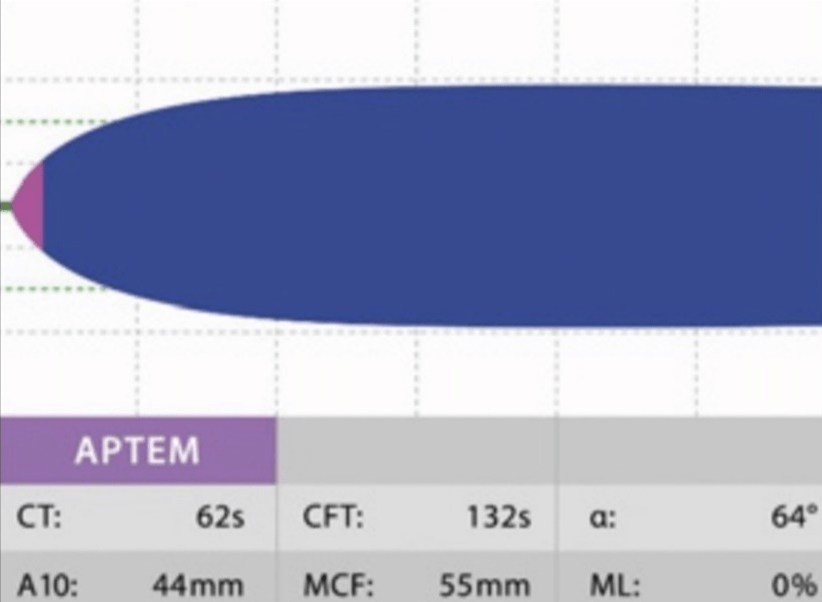
APTEM: This assay employs TF and AP, or tranexamic acid, as reagents in order to activate the EXTEM and inhibit fibrinolysis. It illustrates the contributions of tissue factor, factor VII, platelets, fibrinogen, and antifibrinolytic agents to clot formation and lysis. It is useful to diagnose hyperfibrinolysis by comparing it with EXTEM and monitoring antifibrinolytic therapy (Figure 6).
Figure 6. Normal APTEM18
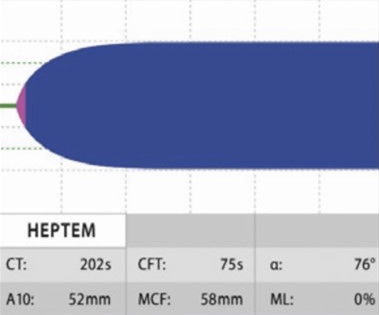
HIPTEM: This test uses lyophilized heparinase to neutralize heparin, and it gives a result that shows if there is any coagulopathy along with heparinization. Since the test was conducted both with and without heparinase, HEPTEM and INTEM are nearly identical in their reference intervals. HEPTEM is best used when a patient is heavily heparinized (such as when they are on bypass), and the doctor wants to know how much residual coagulopathy to expect after the heparin is reversed with protamine. A significantly shorter HEPTEM CT when comparing INTEM and HEPTEM would indicate a heparin effect (i.e., the CT is shortened by adding heparinase) (Figure 7).8
Figure 7. Normal HIPTEM18
Each ROTEM® assay provides a graphical trace that shows the changes in clot elasticity over time. The trace can be divided into four phases: initiation, amplification, stabilization, and lysis. Each phase corresponds to different parameters that describe the kinetics and quality of clot formation and lysis. The most commonly used parameters are:
Clotting time (CT): The time it takes from the start of the assay to the initiation of clot formation. It reflects the initiation phase of coagulation and primarily depends on the concentration and activity of the initiating factors (TF or EL) and the coagulation factors (VII or VIII).
Clot formation time (CFT): This refers to the amount of time between the initiation of clot formation and the amplitude of 20 mm firmness of clot formation. It is a reflection of the amplification phase of coagulation and primarily depends on platelet concentration and fibrinogen activity.
Alpha (α) angle: This is the angle between the baseline and the tangent to the curve at an amplitude of 2 mm. It reflects the slope of clot formation and is mainly influenced by the same factors as CFT.
Maximum clot firmness (MCF): This is the maximum amplitude of the curve. It reflects the stabilization phase of coagulation and primarily depends on platelet concentration and fibrinogen activity.
Maximum lysis (ML): This term is used to describe the decrease in clot firmness following MCF in comparison to MCF. This is the maximum percentage decrease in amplitude from MCF. It reflects the lysis phase of coagulation and primarily depends on the equilibrium between fibrin formation and fibrinolysis. Reduction in clot firmness following MCF in relation to clot stability (ML 15%) or fibrinolysis (ML >15% within 1 hr).
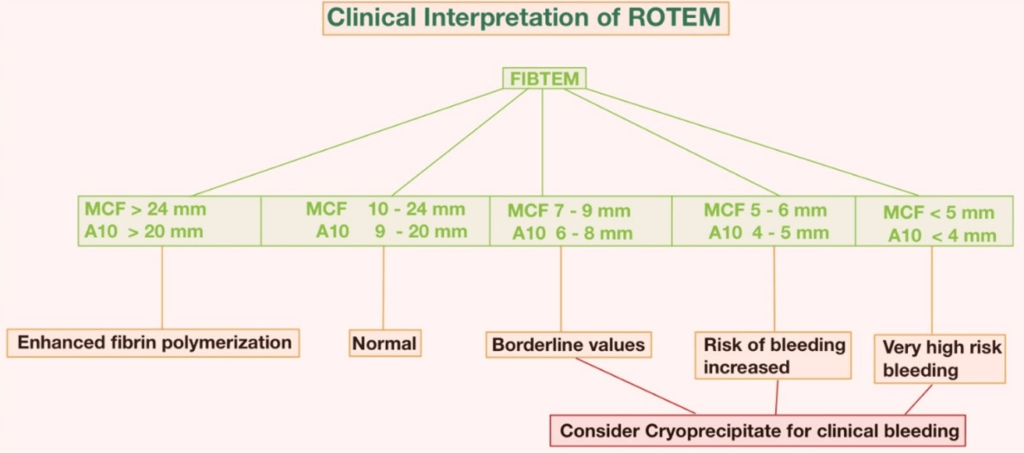
The interpretation of ROTEM® parameters depends on the comparison of the measured values with the reference ranges for each assay. The reference ranges are derived from population-based studies that consider various factors such as age, sex, and ethnicity. The interpretation also depends on the pattern of abnormalities that are observed in different coagulation disorders (Figures 8-10).
Figure 8. Clinical Interpretation of ROTEM®21
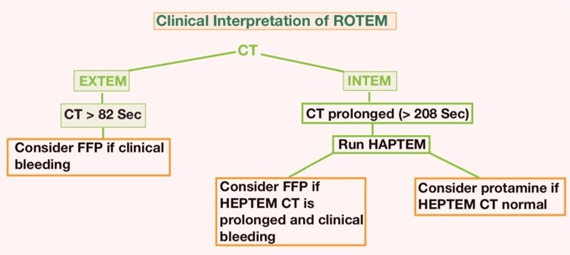
Figure 9. Clinical Interpretation of ROTEM®21
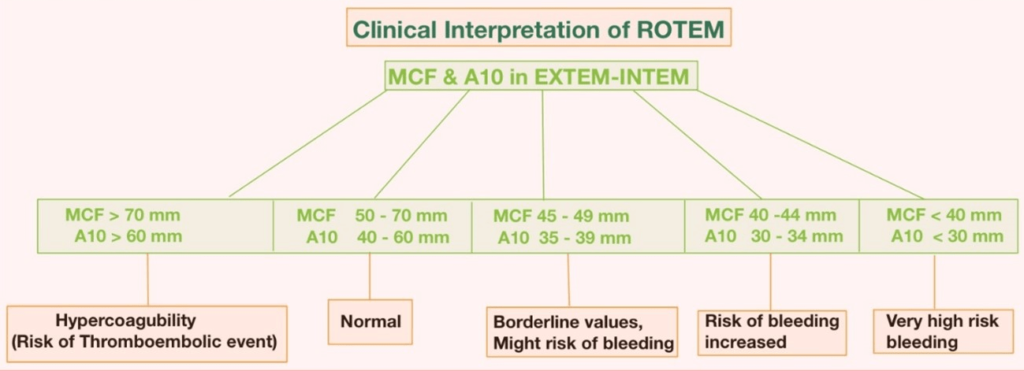
Figure 10. Clinical Interpretation of ROTEM®21
The ROTEM® analysis covers the entire process of whole blood coagulation, from the formation of the first fibrin strands to the maximum firmness of the clot and its lysis. The assessment of the ROTEM® analysis is carried out along the time axis (from left to right): A disturbed activation of coagulation is indicated by a prolonged clotting time. As causes, a factor deficiency or a heparin effect have to be considered. The comparison of INTEM and HEPTEM allows for the specific detection of a heparin effect. An abnormal clot formation is indicated by a prolonged clot formation time (CFT) and/or a reduced clot firmness (A10/MCF). Thus, a disorder of clot polymerization affects the CFT more strongly than the MCF. Therefore, a prolonged CFT in conjunction with a normal A10/MCF points to a polymerization disorder, whereas a reduced A10/MCF in conjunction with a normal CFT points to a lack of clottable substrate (fibrinogen and/or platelets). Fibrinolysis is detected by the lysis of the clot (ML >15%) or by the finding of a better clot formation (shorter CFT, greater MCF) in APTEM as compared to EXTEM. Hyperfibrinolysis is confirmed if it occurs in APTEM and displays the typical hyperfibrinolysis pattern (spindle-shaped, total lysis of the clot firmness).
HEPTEM, APTEM and FIBTEM
To understand HEPTEM and APTEM, you need to compare them to INTEM or EXTEM. Heparin has an effect if the time it takes for blood to clot in HEPTEM is shorter than in INTEM. In the EXTEM, INTEM, or FIBTEM tests, fibrinolysis is shown by the “spindle” shape of the TEMogram. After an optional hyperfibrinolytic treatment, the return of a normal TEMogram shape in the APTEM assay confirms the fibrinolysis and enables evaluation of the patient’s clot quality. A decreased MCF in FIBTEM indicates a decreased fibrinogen concentration and/or clot polymerization inhibition. There are often differences between FIBTEM and laboratory measurements of fibrinogen because FIBTEM is much more sensitive to clot polymerization problems than traditional laboratory tests.
In addition, a few important points that will help to conclude the assay are listed below.
1. A CT INTEM prolongation suggests either a lack of intrinsic pathway coagulation factors or a heparin effect.
2. A CT EXTEM prolongation suggests a lack of coagulation factors in the extrinsic pathway.
3. A reduced A10 level in either INTEM or EXTEM indicates insufficient clot firmness. This is because of decreased levels of platelets, fibrinogen, and factor XIII.
4. A reduced MCF level in INTEM or EXTEM is another sign of a weak clot. This is due to low-levels of platelets, fibrinogen, and factor XIII.
5. A reduced MCF in FIBTEM indicates an insufficient fibrin level, which is necessary for clot firmness.
6. More than 15% increases in ML in INTEM, EXTEM, and FIBTEM all point to hyperfibrinolysis (Figures 11-14).
Figure 11. ROTEM with Thrombocytopenia or Poor Platelet Functions18
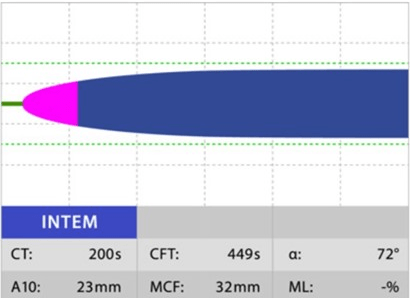
Figure 12. ROTEM in Patient with Low Fibrinogen18
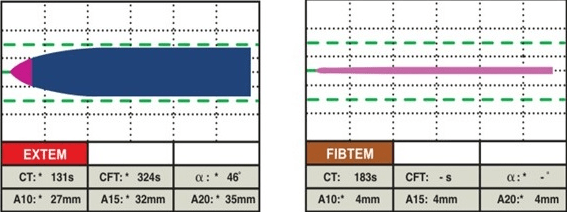
Figure 13. ROTEM Under the Effects of Heparin18
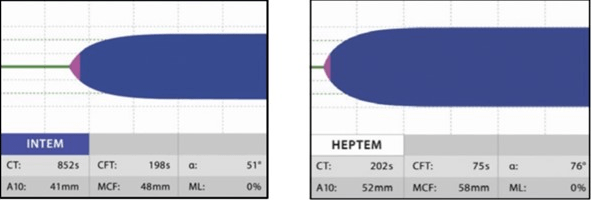
Figure 14. ROTEM shows Hyperfibrinolysis18
In general, there are two main patterns of abnormalities that can be seen in ROTEM® assays:
Hypocoagulability: This is characterized by a prolonged CT, CFT, or α, a decreased MCF, or an increased ML, indicating a reduced ability to form or maintain a stable clot. This can be seen in conditions such as coagulation factor deficiency or inhibition, platelet deficiency or dysfunction, fibrinogen deficiency or dysfunction, or hyperfibrinolysis.
Hypercoagulability: This is characterized by a shortened CT, CFT or α, an increased MCF, or a decreased ML, indicating an increased ability to form or maintain a stable clot. This can be seen in conditions such as coagulation factor excess or activation, platelet excess or activation, fibrinogen excess or activation, or hypofibrinolysis.
Some conditions may present with a mixed pattern of hypocoagulability and hypercoagulability, such as disseminated intravascular coagulation, liver failure, or sepsis.
The severity of the abnormalities can be graded according to the following criteria:
Mild: CT: 80-120 s; CFT: 80-240 s; α: 47-83°; MCF: 50-70 mm; ML <15%
Moderate: CT: 120-180 s; CFT: 240-360 s; α: 30-47°; MCF: 35-50 mm; ML: 15-30%
Severe: CT >180 s; CFT >360 s; α <30°; MCF <35 mm; ML >30%
ROTEM® use is indicated and its beneficial to guide transfusion in patients with an increased risk of haemorrhage, as outlined below:
• Massive transfusion protocol activation for trauma, obstetric or postpartum haemorrhage, or large GI bleeding
• Operating room resuscitation and stabilization of patients on cardiopulmonary bypass
• Unknown bleeding causes in ICU patients, especially those in acute or acute-on-chronic liver failure
• Unclear or excessive bleeding causes for those on anticoagulation therapy (e.g., ECLS).
• Pre-operative optimization of liver patients needing procedures
• Concern for hyperfibrinolysis states (e.g., DIC or coagulopathy of trauma).
Figures 15 and 16 depict the interpretation of the ROTEM® algorithm in PPH and Trauma. In addition, a link to the NHS ROTEM® interpretation algorithm is provided below.22 These algorithms can be used to guide blood transfusions and fix specific deficiencies (FFP, platelets, RBC, and cryoprecipitate) in cases of massive bleeding in PPH, trauma, and major vascular surgeries.
Figure 15. ROTEM Algorithm in PPH19
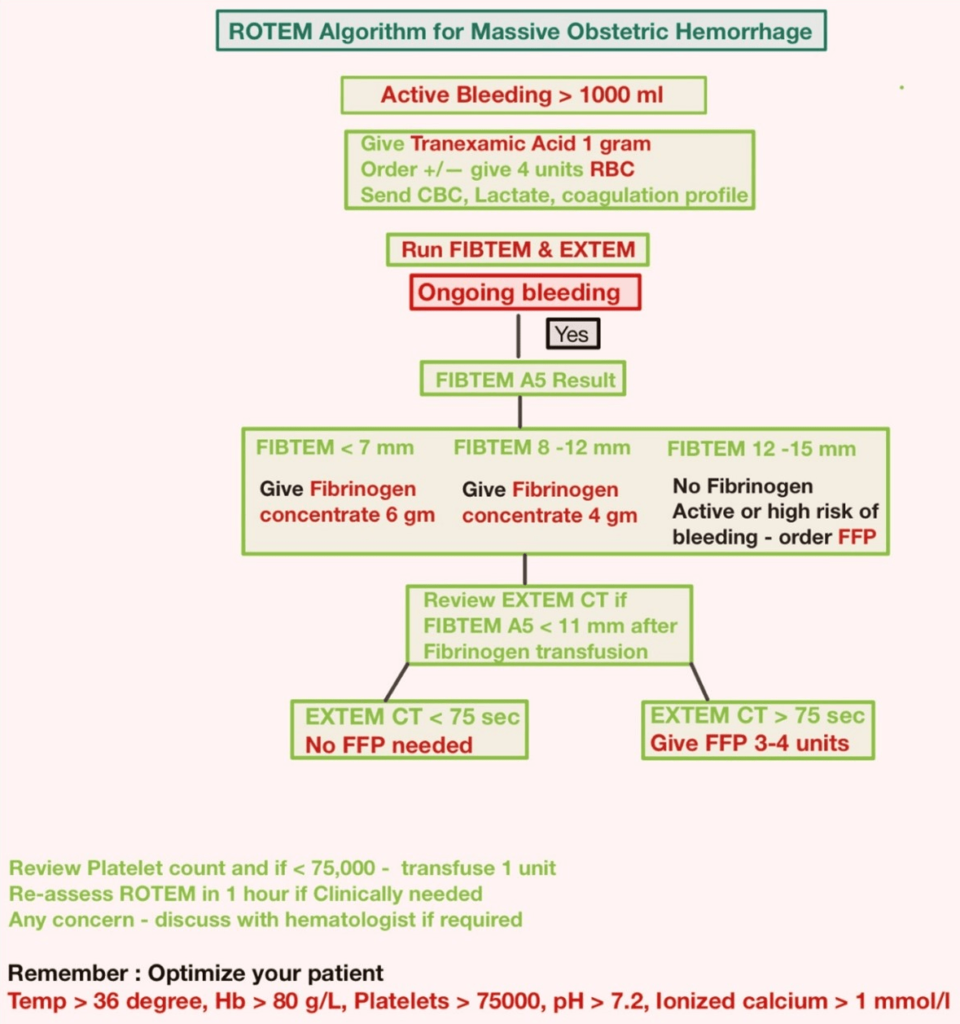
Figure 16. ROTEM-guided Treatment Algorithm: Managing Trauma-induced Coagulopathy and Diffuse Microvascular Bleeding20
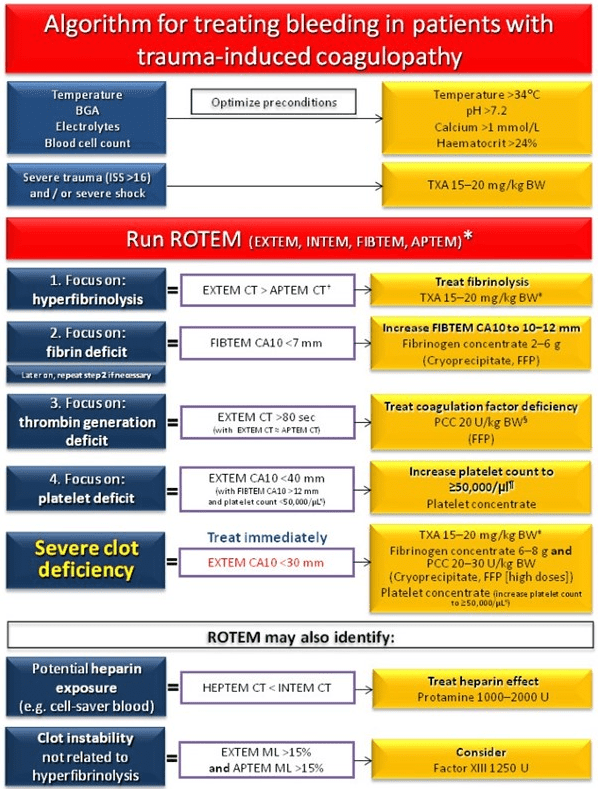
Normal values: EXTEM/APTEM coagulation time (CT): 38-79 seconds; EXTEM/APTEM clot amplitude at 10-minutes (CA10): 43-65 mm; EXTEM/APTEM maximum lysis (ML) <15%; FIBTEM CA10: 7-23 mm; INTEM CT: 100-240 seconds. CA10, clot amplitude at 10-minutes; BGA, blood gas analysis; BW, body weight; Ca, calcium; CT, clotting time; FFP, fresh frozen plasma; ISS, injury severity score; MCF, maximum clot firmness; ML, maximum lysis; PCC, prothrombin complex concentrate; TXA, tranexamic acid.
Adapted from Schöchl H20
ADVANTAGES OF ROTEM®
ROTEM® has several advantages over conventional coagulation tests, such as:
• It provides rapid results within minutes.
• It can detect both hypercoagulability and hypercoagulability.
• It measures global coagulation function in whole blood samples.
• It can guide targeted transfusion therapy based on evidence-based algorithms.
• It can reduce transfusion requirements and improve patient outcomes.
LIMITATIONS OF ROTEM®
It requires specialized equipment and trained personnel.
• It is affected by several factors such as technique, sample quality, anticoagulants, haemodilution, hypothermia, and inflammation.
• It does not provide detailed information about specific coagulation factors or inhibitors.
• It does not differentiate between reversible and irreversible causes of coagulopathy.
• It may not detect mild or early stages of coagulopathy.
• It may not detect aspirin, clopidogrel, or any antiplatelet drug.
• It may not detect Von Willebrand disease.
DISCUSSION
A more thorough analysis of the coagulation system in liver cirrhosis has been proposed using ROTEM®, a comprehensive blood coagulation assay that offers insight into global hemostatic capabilities. Rotational thromboelastometry (ROTEM) improves hemostasis assessment when compared to conventional coagulation testing in ACLF and non-ACLF patients, according to a study by Seeßle et al.9 This prospective cohort study examined fifty-five non-ACLF patients and twenty-two ACLF patients, and it concluded that hemostasis in these patients showed advantages of ROTEM® over CCT. When predicting bleeding events in the ACLF group, the prognostic parameters A10 and MCF are appropriate. In a different study by Al Moosawi et al10 ROTEM® was used in liver transplant surgery on 197 patients (138 in the post-ROTEM® group and 59 in the pre-ROTEM® group), and the results showed that EXTEM CT was seen in 95.3% and 93% of patients with INR of 1.3-1.8 and up to 3, respectively. After adjusting for red cell utilization and coagulation testing, ROTEM®-guided transfusion in liver transplant surgeries was associated with less plasma transfusion.
To determine the sensitivity and specificity of ROTEM® to detect coagulopathy in PPH, Bell et al11 conducted a prospective study on 521 women. They concluded that ROTEM® Sigma FIBTEM A5 can detect fibrinogen below 2 g/L and guide targeted fibrinogen replacement. Another study, Four Years’ Experience of an ROTEM® Guided Algorithm for Treatment of Coagulation Disorder in Obstetric Haemorrhage, by McNamara et al12 showed improved clinical outcomes for selective use of fibrinogen concentrate in the treatment of coagulopathy detected using ROTEM®.
According to Brill et al’s13 study; “The Role of TEG and ROTEM® in Damage Control Resuscitation” viscoelastic testing, including TEG and ROTEM®, has been shown to more accurately predict the need for massive transfusions and detect trauma-induced coagulopathy than clinical judgment or traditional coagulation tests. Furthermore, TEG and ROTEM® can guide resuscitation to combat specific physiologic coagulation derangements within minutes of a patient’s arrival and are keys to increasing survival. Salehi et al14 did another Cohort study on ROTEM®-guided blood transfusions in Trauma patients. They found that the use of fibrinogen went up, but this did not change the mortality rate and didn’t make a difference in the administration of red blood cells, fresh frozen plasma, platelets, and cryoprecipitate. Another cohort study conducted on 301 patients by Smith et al15 on the prediction of mortality in severe trauma patients using ROTEM® concluded that a low APTEM α angle, an elevated APTEM clot formation time, and a high APTEM clotting time significantly predicted mortality, while abnormal PT and PTT did not appear to be associated with increased mortality in this patient population.
Veigas et al16 conducted a systematic review on the ROTEM® values for the diagnosis of coagulopathy, prediction, and guidance of blood transfusion, and the prediction of mortality in trauma patients. The inclusion criteria were met by 13 observational studies involving 2835 adult trauma patients. There were four retrospective studies and nine prospective ones. No randomized controlled trials existed. Most of the evidence suggests that abnormal EXTEM and FIBTEM clot amplitude (CA5, CA10) or maximal clot firmness (MCF) diagnose coagulopathy and forecast the need for blood transfusions and mortality, according to the study’s findings. Mortality was also correlated with the presence of fibrinolysis (abnormal lysis index (LI30) or maximum lysis (ML)). ROTEM® thus, may be of value in the early management of trauma patients.
Deppe et al17 performed one of the metanalyses on 8332 patients undergoing cardiac surgery for thromboelastography/thromboelastometry-based point-of-care coagulation management. The study concluded that TEG/ROTEM®-based coagulation management significantly lowers the rate of re-exploration after cardiac surgery, the incidence of post-operative acute kidney injury (AKI), and thromboembolic events in cardiac surgery patients.
CONCLUSION
ROTEM® analysis is unquestionably superior to standard coagulation tests for identifying patients with thrombotic complications. In organ transplantation (liver, heart, lung, and renal), cardiovascular surgery, postpartum hemorrhage, and trauma, ROTEM® is effectively utilized to assess clinical conditions and guide transfusion. ROTEM® can help diagnose and treat perioperative coagulopathy, reduce unnecessary transfusions, and improve patient outcomes. However, ROTEM® should be used cautiously and in conjunction with a clinical history and physical examination. Still, more research should be conducted to improve ROTEM® compliance and optimize ROTEM®-guided transfusion to reduce blood product overuse in trauma patients and PPH.14
FUNDING
None.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.





















