INTRODUCTION
Healthcare-associated infections (HAIs) are a major concern in today’s era of medical advancement. According to World Health Organization (WHO), effective infection control and prevention can reduce HAIs by at least 30%.1 HAIs are one of the leading causes of morbidity and mortality worldwide. Prolonged hospital stay, long-term disabilities, increased antibiotic resistance, increased financial burden are some of the implications of HAIs. Catheter-associated urinary tract infections (CAUTI) is the most common HAI and most of them are caused by indwelling urinary catheters.2 CAUTI is almost always preceded by bacteriuria which develops with a frequency of 3-6% per day in a catheterised person.3 Patients usually present with local and systemic symptoms of bacteriuria such as urgency, dysuria, abdominal pain and fever. The main causal agents of CAUTI are commensal perineal flora. Most CAUTIs are monomicrobial commonly caused by E.coli, Pseudomonas aeruginosa, Enteroccocci, candida, klebsiella and enterobacter. 4 CAUTIs can lead to cystitis, pyelonephritis and bacteremia. Bacteremia can in turn lead to septicaemia and septic shock which carries high mortality rate. Thus early prevention of CAUTI is of utmost important. High-risk individuals for acquiring CAUTI include those patients who require prolonged catheterisation and long hospital stay, patients with diabetes mellitus, Fournier’s gangrene etc., Several urinary catheters have been specially designed to reduce the risk of infections and CAUTI. These include catheters with antiseptic and antimicrobial compounds such as silver ions, antibiotics and noble metal alloys. Here we describe our experience with the use of bactiguard infection protection (BIP) foley’s catheter used in a patient with fournier’s gangrene.
CASE REPORT
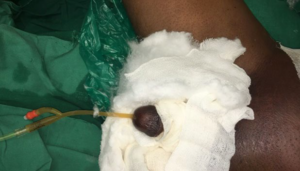
This study was conducted in Department of Plastic Surgery, JIPMER, Pondicherry, India in a tertiary care centre in the month of August 2019. Informed consent was taken from the participant. A 16 Fr BIP Foley’s catheter (Latex) was used in a 55-yearold patient with Fournier’s gangrene and Type 2 diabetes mellitus (T2DM) (Figures 1 and 2). Inserted by trained staff under proper aseptic precaution the silicone BIP catheter was retained for 10- days. Urine routine microscopic examination was done prior to catheterisation which showed no pus cells. Patient was managed by serial debridements and supportive care. Urine routine was done weekly once as part of routine investigation. Repeat urine routine examination again showed no pus cells. Patient’s condition suddenly deteriorated on the 10th day of hospitalisation and he succumbed due to pulmonary embolism. Throughout the stay in the hospital patient did not develop features of urosepsis.
Figure 1. BIP Cathete

Figure 2. Patient with BIP Catheter in Situ
RESULTS
The study results showed that during his stay in the hospital patient did not develop catheter-associated urinary tract infection (Table 1).
| Table 1. Urinalysis (Before Catheterisation) |
| Chemical Examination |
| Glucose |
++ |
| Protein |
+ |
| Microscopic Examination |
| RBC(/HPF) |
Absent |
| WBC(/HPF) |
Absent |
| Epithelial Cells (/HPF) |
Absent |
| Bacteria |
Absent |
| Crystal |
Absent |
| Cast |
Absent |
Patient’s urine routine examination did not reveal any pus cells. There were no symptoms or signs of urinary tract infection in the patient (Table 2).
| Table 2. Urinalysis (1-Week Post-catheterisation) |
| Chemical Examination |
| Glucose |
++ |
| Protein |
+ |
| Microscopic Examination |
| RBC(/HPF) |
Absent |
| WBC(/HPF) |
Absent |
| Epithelial Cells (/HPF) |
2-3 |
| Bacteria |
Absent |
| Crystal |
Absent |
| Cast |
Absent |
DISCUSSION
Healthcare-associated infections are widely researched topics worldwide with urinary tract infections coming in the forefront and prolonged catheterisation being the harbinger of urinary tract infections. Various methods have been devised to reduce the risk of catheter-associated urinary tract infections. It is well-established that the duration of catheterisation is directly linked to the development of urinary tract infections. With a catheter in place the daily risk of developing urinary tract infection (UTI) ranges from 3-7%.5 CAUTI occurs when urethral catheters inoculate organisms in the bladder and cause colonization due to providing a medium for bacterial adhesion and mucosal irritation. Urinary catheter is the most important risk factor for bacteriuria.6,7 Biofilm on the urinary catheter is the central factor in pathogenesis of CAUTI, many scientists seek to alter the catheter surface in order to inhibit biofilm formation. No surface can resist biofilm formation indefinitely in the urinary tract, but impeding biofilm formation may suffice if the catheter is intended for short-term use.8 As biofilm formation is central to the pathogenesis of CAUTI, novel methods to hinder or alter biofilm formation on the surface of urinary catheters might assist in prevention of CAUTI. BIP Foley catheters are designed to reduce and delay morbidities related to CAUTI (bacteriuria, symptomatic infections, bacteremia, urosepsis). The BIP Foley catheters are approved for transurethral and suprapubic use for up to 90-days. The BIP technology is based on a very thin noble metal alloy coating, consisting of gold, silver and palladium, firmly attached to medical devices. When in contact with fluids, the noble metals create a galvanic effect. The galvanic effect creates a microcurrent that reduces microbial adhesion to the catheter material, which decreases the risk for biofilm formation leading to infections.9 The amount of noble metal is very low and below the safety limits for each metal and there is no release of any toxic or pharmacological quantities. The bactiguard coating is environmentally friendly and requires no special procedures for handling, use or disposal. The bactiguard solution is unique, tissue-friendly and safe for patient use. The BIP Foley Catheters are available in both latex and silicone.
They are also coated with hydrogel to reduce friction at insertion. The cost of the catheter is approximately 800 Indian Rupees (INR) compared to approximately 120 INR for a normal Foley’s catheter. In our study, we describe our experience regarding the use of BIP catheter. Our patient even though a high-risk candidate for development of UTI as he was a diabetic with Fournier’s gangrene did not develop any features of CAUTI during 10-days duration of catheterisation. Limitations of the study being, it is a single case, single centre study. The present study is a preliminary study which requires randomised controlled multicentric trials with statistical analysis to further substantiate the role of BIP technology in reducing the risk of CAUTIs.
CONCLUSION
The BIP technology catheter is claimed to be effective in reducing the risk of catheter-associated urinary tract infections especially in high-risk individuals with prolonged catheterisation but it requires further testing. We found no CAUTI in one patient after 10-days of catheterisation.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







