INTRODUCTION
Minimally Invasive Esophagectomy (MIE) is becoming a standard surgical method for treating esophageal cancer in Asia. Its outcomes are comparable to those of open surgery. Furthermore, MIE has fewer pulmonary complications and less pain.1,2
The da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) was granted United States Food and Drug Administration approval in July 2000; robotic techniques are increasingly being adopted in America for many types of laparoscopic surgery. The benefits of a robotic approach are three-dimensional vision; camera stability; instruments with a high degree of dexterity, precision, and control; and a 3rd arm for self-assistance.
Studies3,4 have described a robotic approach to esophageal surgery when treating achalasia and hiatal hernia; however, experience with Robot-Assisted MIE (RAMIE) is still limited. Several series5,6,7,8,9,10 have reported variable hybrid approaches for RAMIE, but only two11,12 describe its surgical details and outcomes.
We report our first experience of complete RAMIE with a four-arm robotic platform, and describe technical modifications made to overcome specific challenges. Short-term surgical outcomes and complications encountered are discussed.
PATIENTS AND METHODS
Patient Selection
We enrolled 11 patients with esophageal cancer. All had undergone a RAMIME using a combined thoracoscopic and laparoscopic approach. All patients underwent preoperative staging and evaluation, which included a medical history and physical examination, an upper gastrointestinal endoscopy and biopsy, a bone scan, computed tomography of the chest, and abdominal and endoscopic ultrasound evaluations. Patients with T2 tumors or greater, or nodal involvement, or both, were referred for induction chemoradiation therapy. A waiver of informed consent for retrospective studies was granted by our institutional review board.
All 11 operations were performed by the same two attending thoracic surgeons with advanced experience in MIE (YCS, YF). Most of the operations were assisted by nursing and anesthesiology staff experienced in non-robotic minimally invasive esophageal resections.
Data Collection
Patient demographic characteristics, outcomes, and complications, graded in accordance with the Common Terminology Criteria for Adverse Events version 4.0,13,14 were retrospectively collected by reviewing the patients’ charts. Intraoperative data were obtained from the operative record.
Operation Technique: Thoracoscopic Phase
RAMIE and non-robotic MIE techniques from the Mayo Clinic and Memorial Sloan-Kettering Cancer Center (MSKCC) were used to modify previously published standardized techniques11 and create the technique we used. Our team included 1 attending surgeon (YCS or YF) and an assistant (a fellow or resident) seated at the bedside. For the first few cases, we requested that our fellow (JKC), who is familiar with MIE procedures, be at the bedside. After the procedure had been standardized, and when the residents better understood it, rotating residents replaced JKC.
Patient Positioning and Port Placement
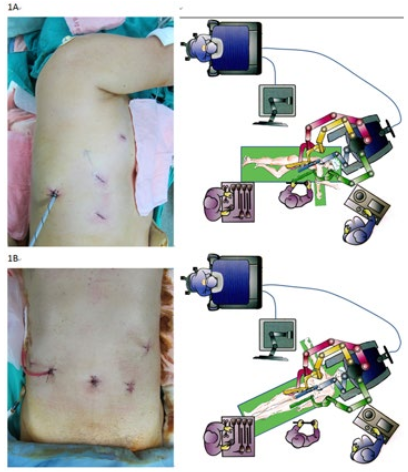
The patient was first placed in the standard left-lateral decubitus position. The table was then rotated leftward to put the patient into a semiprone position, which kept the operation field clear. The robot approached the patient about 45° dorsally from the cranial midline. Port placement, operating layout, and the robot-cart trajectory over the right shoulder are depicted in figure 1. A transparent laparoscopic port (Kii Fios First Entry; Applied Medical, Rancho Santa Margarita, CA, USA) was inserted using a video thoracoscopic guide at the anterior axillary line of the 6th Intercostal space (ICS) for the camera. This prevented the camera from colliding with the iliac crest. CO2 insufflation was then begun at a pressure of 8 mmHg. The 2nd arm was put in the 8th ICS, the midaxillary line. The 3rd arm was put in the 10th ICS, the paraspinal space. It contributed to the lung retraction and reduced the assistant’s work. The 1st arm was put in the 4th ICS, the anterior axillary line. A 12 mm assistant port was placed at the site of the diaphragmatic insertion, the anterior axillary line, between the camera and the 2nd arm. The robotic camera was introduced facing 30° downward.
Figure 1: (A) Thoracoscopic phase room setup and port placement; (B) laparoscopic phase room setup and port placement.
Esophageal Mobilization
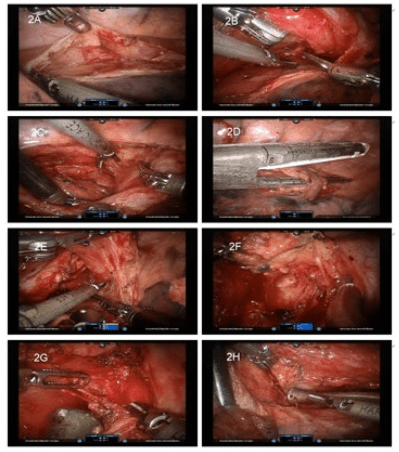
The important steps of the thoracoscopic phase are illustrated in figure 2. An ultrasound shears (Harmonic Scalpel; Ethicon, Cincinnati, OH, USA) or unipolar spatula was used in the 1st robotic arm; a bipolar fenestrate grasp was used in the 2nd arm; and a double fenestrate grasp was used in the 3rd arm for lung retraction. Dissection was begun at the level of the inferior pulmonary vein and then continued ventral to the esophagus along the pericardium to the left pleura (Figure 2A) and dorsal to the esophagus along the spine and aorta to the left pleura. When the thoracic duct was found midway, it was ligated using a ligation system (Hem-o-lok; Teleflex, Research Triangle Park, NC, USA). When the dissections conjoined at the contralateral side, the esophagus was retracted with the 3rd arm (Figure 2B). Then the instruments in the 1st and 2nd robotic arms were exchanged. A tension plane was created and transected using the 2nd-arm energy-instrument (Figure 2C). The subcarinal lymph nodes were removed in this manner. Then the azygos vein was looped and transected with an endocutter (Echelon; Ethicon, Cincinnati, OH, USA) (Figure 2D). The posterior stump can be sutured to the chest wall for a better view. The right bronchial artery and vagus nerve were transected at this level. The left pleura and aortic arch were reached as a left boundary (Figure 2E). The dissection could easily exceed the thoracic inlet. Recurrent laryngeal nerve injury should be avoided here. Because the harmonic scalpel is usually too short here, the instruments in 1st and 2nd robotic-arm were exchanged. When the cranial part was finished, we turned to the hiatal region (Figure 2F). After a circular dissection of the hiatus, the cardia portion of the stomach was visible.
Lymph Node Dissection
The inferior pulmonary ligament and subcarinal lymph node were removed during the esophageal dissection. When dealing the paratracheal lymph nodes, the 3rd arm was useful for retraction. When dissecting between the vagus nerve and superior vena cava, the medial side of the aorta could be reached. Take care not to injure the left recurrent laryngeal nerve (Figure 2G). Dissecting cranially between the trachea and vagus nerve cranially led us to the right recurrent laryngeal nerve (Figure 2H).
Figure 2: Thoracoscopic phase procedures.
Operation Technique: Laparoscopic Phase
Patient positioning and port placement
Patients were placed supine on the operating table, with their right arms abducted at about 60°. Their left arms were adducted for an easier cervical approach. The table was turned to reverse Trendelenburg position to allow the intestine to move caudally. The cervical approach was used first because it is difficult to do so when the robot is docked. The esophagus was looped and hung with a rubber tube for later use.
The robot approached directly over the patient from the midline. Port placement, operating layout, and robotic cart trajectory are shown in figure 1. The camera port was made at the umbilicus. To enter the peritoneal cavity the open method was used, and then a balloon port (Kii Balloon Blunt Tip; Applied Med, Rancho Santa Margarita, CA, USA) to control air leaks. The peritoneum was distended using standard CO2 insufflation. A standard 10 mm, 0° laparoscope was used for the initial inspection. The other ports’ positions were determined using the camera rather than marks on the skin. A right-lateral, subcostal port for the 3rd arm was first placed just above the intestinal plane. A mid-clavicular port for the 2nd arm located midway between the camera port and the 3rd arm port was then made. A left-lateral subcostal port for the 1st arm was made. Finally, a left mid-clavicular 12 mm port was made for the assistant. This port was about 3-5 cm caudal to the umbilicus to avoid collision with the camera arm. If the patient has a previously made feeding jejunostomy, this port is placed lateral to the feeding jejunostomy stoma.
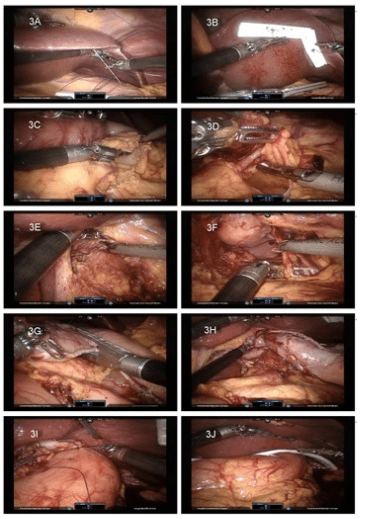
The important steps of the laparoscopic phase are illustrated in figure 3. After docking, we began the liver retraction. A needle driver was used in the 1st arm, a bipolar fenestrate grasp in the 2nd arm, and a double fenestrate grasp in the 3rd arm. A handmade liver retractor14 was inserted into the peritoneal cavity. A straight needle was used to puncture at 2cm from the liver boarder to the abdominal wall around the xyphoid region (Figure 3A). Use the straight needle to bring the strings outside peritoneum and hang up a segment of JP (Jackson-Pratt) drain as a liver retractor. Two liver retractors were used to afford enough retraction force (Figure 3B). The strings were then pulled to hang up the liver with adequate tension.
Figure 3: Laparoscopic phase procedures.
Greater curvature mobilization
A 30° downward scope was used. A bipolar fenestrate grasp was in the 1st arm and ultrasound shears in the 2nd arm. The double fenestrate grasp in the 3rd arm was of great help for retracting the stomach. At first, the lesser sac was entered by dissecting the omentum. The dissection was performed 2 cm away from the right gastroepiploic artery to prevent an incidental thermal injury. When we entered the lesser sac, we inserted the double fenestrate grasp into it and retracted the stomach together with the right gastroepiploic artery (Figure 3C). With this approach, we could do a safe dissection. When the left gastroepiploic artery was met, we exchanged the instruments
in the 1st and 2nd arms to promote short gastric artery dissection. The short gastric of the gastric fundus, however, was very difficult to approach without grasping. Therefore, we left the area and looked back to the pylorus portion. Using the 3rd arm to retract the stomach upward, the dissection of the pylorus portion was easily done.
Hiatal and retrogastric dissection
The left gastric vascular pedicle in the lesser curvature (Figure 3D) was found and transected with an endocutter. The hepatic trunk lymph node and hiatal crus dissections were performed (Figure 3E) with the 3rd arm retracted. After the hiatal dissection, it was easy to approach the short gastric arteries of the fundus portion via the lesser curvature (Figure 3F). We then finished the gastric mobilization.
Gastric tubularization
We introduced a new approach to form a gastric tube. To prepare for gastric tubularization, we used the 3rd arm to caudally retract the stomach via the lesser curvature. This approach elongated the stomach and opened the gastric rugae. We transected the lesser curvature mesentery at the junction of the right and left gastric arteries, and then transected the stomach with the endocutter to make a 3 cm wide tubular conduit (Figure 3G). When the endocutter approached the hiatus, we did not separate the hiatus and the conduit but simply left a 1 cm wide attachment there. Later, the lesser curvature could be pulled up together with the gastric tubular conduit (Figure 3H). To preclude the possibility of a blood supply compromise, we did not reinforce the sutures over the staple line; there was no leakage from this region.
Cervical anastomosis
By pulling the esophagus out via the cervical wound, the tubular gastric conduit and the remnant of the gastric cardia came out together. A correct axis was ensured with laparoscopic monitoring. A suture was made to prevent conduit torsion (Figure 3I). A JP drain was placed in the subhepatic region with the tip in the left subphrenic region via the 2nd arm port (Figure 3J). After the robot was undocked, the cervical anastomosis was made in a side-to-side pattern with the endocutter. The opening of the anastomosis was also closed with the endocutter. No pyloroplasty was performed, and no feeding jejunostomy was made in patients who had none preoperatively.
RESULTS
Demographic Characteristics
Eleven consecutive male patients underwent RAMIME between May 2012 and March 2013 (median age: 57 years; range: 45-83) (Table 1). Nine patients had squamous cell carcinoma, and 2 patients had adenosquamous cell carcinoma: 4 patients had cancer in the upper 1/3 of the esophagus, 5 in the middle 1/3, and 2 in the lower 1/3. A McKeown (three-field) approach was used for all patients, and 10 patients underwent neoadjuvant chemoradiation therapy.
| Table 1: Patient demographics (n = 11) and data summary. |
| Variable |
Number of Patients (%) |
| Age in Years [median (range)] |
57 (45-83)
|
| Gender (male) |
11 (100)
|
| Histological Result |
| Squamous Cell Carcinoma |
9 (92)
|
| Adenocarcinoma |
0 (0)
|
| Adeno-Squamous Cell Carcinoma |
2 (18)
|
| Overall Stage, TN Clinical Stage |
| Stage IIB |
| T3N0 |
2 (19)
|
| Stage IIIA |
| T3N1 |
6 (53)
|
| T3N2 |
2 (19)
|
| Stage IV |
|
| T3N0M1 |
1 (9)
|
| Induction Therapy |
| Chemotherapy and Radiation |
10 (91)
|
| Lymph Nodes Resected
[median (range)] |
28 (9-39)
|
| Extent of Resection |
| R0 |
10 (91)
|
| R1 |
1 (9)
|
| Length of Stay in Days
[median (range)] |
18 (14-36)
|
| All data are given as number (%) unless otherwise indicated.
R0: gross and microscopic margins negative; R1: gross margin negative/microscopic margin positive. |
Perioperative Outcomes
Median operative time (incision through wound closure, including docking and repositioning time) was 795 min (range: 635-975 min); Median thoracoscopic console time was 270 min (range: 135-330 min); and median laparoscopic console time was 160 min (range: 150-260 min). Ten patients had a complete macroscopic and microscopic (R0) resection. Median blood loss was 300 cm3 (range: 100-650 cm3); median number of lymph nodes harvested was 28 (range: 9-39); median hospital stay was 18 days (range: 14-36 days); median intense care unit stay was 3 days (range: 3-17 days); and median number of ventilation days was 1 (range: 1-17 days).
Conversions
No patients were converted to an open, non-robotic laparoscopic, or thoracoscopic approach.
Complications
Four patients had grade III or greater complications.
Two had pneumonitis and thus required a longer intubation time. One had an infection related to an implantable venous access device (Port-a-Cath): when antibiotic treatment was stopped, the patient had a fluctuating fever that subsided after the device was removed. Finally, one patient died of hepatic failure secondary to liver cirrhosis on postoperative day 16. The most common complication was pneumonitis (5 patients). The rate of grade II or greater anastomotic leak was 0% (Table 2).
| Table 2: Patient complications by Common Terminology Criteria for Adverse Events version 4.0. |
| Grade, Complication |
Number of Patients |
| Grade I |
|
| Anastomotic Leak |
1
|
| Delayed Gastric Emptying |
2
|
| Chylothorax |
1
|
| Grade II |
| Atrial Fibrillation |
1
|
| Pneumonitis |
3
|
| Wound Infection |
1
|
| Chylothorax |
1
|
| Recurrent Laryngeal Nerve Palsy |
1
|
| Grade III |
| Pneumonitis |
2
|
| Catheter Related infection |
1
|
| Grade V |
| Hepatic Failure |
1
|
DISCUSSION
MIE has been popular in Asia for the past decade, and reports1,2 indicate that its popularity is increasing worldwide as well. To a lesser extent, hybrid RAMIE approaches, usually thoracoscopic, have also become popular. The advantages of the robotic approach-three-dimensional vision, camera stability, and instruments with a high degree of freedom have been emphasized.5,7,10,11
There are only two other published reports of complete RAMIEs using a combined thoracoscopic and laparoscopic approach. In 2007, Kernstine et al.11 reported 8 complete RAMIMEs with a three-arm platform and 6 hybrid RAMIMEs. The thoracoscopic portions were done in a prone position. In the complete RAMIME group, there was one conversion to a thoracotomy. The median operative time was 672 min (range: 570-780 min). The incidence of major morbidity was 29%: 2 anastomotic leaks, 1 thoracic duct leak, and 2 cases of vocal cord paralysis. One patient with persistent aspiration pneumonia died 90 days post-surgery.
In 2013, Sarkaria et al.12 reported the first series of complete RAMIEs with a four-arm robotic platform. Seventeen Ivor Lewis RAMIEs and 4 RAMIMEs have been reported. A left lateral decubitus position was used in the procedure. In contrast to Kernstine et al.11 Sarkaria et al. used the prone position, which, although it has some advantages in lung retraction and requires clearing blood from the surgical field, also presents difficulties when it is necessary to convert to an open procedure. Five (24%) of 21 patients were converted to laparoscopic or thoracoscopic approaches. Another 5 were converted to open surgery. The median operative time was 556 min (range: 395-626 min) the incidence of major morbidity was 24%, including anastomotic leak (3 patients: 14%), respiratory failure (2 patients: 10%), pulmonary embolism (2 patients), and vocal cord paralysis (1 patient: 5%). One patient with respiratory failure secondary to anastomotic leak and tracheobronchial fistula died 70 days post-surgery.
The present study is the first reported series of RAMIMEs using a four-arm robotic platform and a modified semi-prone position. The procedure was developed and modified from the minimal invasive McKeown esophagectomy we have regularly performed for the past 7 years. One key difference between the prone and the left lateral decubitus positions is the facility of the 3rd robotic arm. In the prone position, there is not enough room to use a 3rd robotic arm. Although we can rely in part on gravity to allow us to retract the lung, the 3rd robotic arm in the left lateral decubitus position offers better retraction force and dramatically decreases the assistant’s work. Because of this, we placed the patient in the left lateral decubitus position to get enough space for the 3rd arm. Then the operation table was rotated leftward to create a semi-prone position. With this modification, we had enough room to dock the 3rd robotic arm and still use gravity to aid the lung retraction.
The extent of the lymphadenectomy and the rate of complete resection were comparable to those in Kernstine et al.11 and Sarkaria et al.12 both for open surgery and for MIEs. No patient had positive microscopic margins (R1) on the transection edge. Although a pathologist said that there were positive radial margins in 2 patients, the attending surgeons thought there was such a margin in only 1 patient: we found a pleural metastasis in one patient and resected that lesion with a clear safe margin.
We found an overall incidence of major morbidity and mortality, including the rate of anastomotic leak, similar to that in other series, both for open surgery and for MIEs. One patient, with a history of HBV infection but not liver cirrhosis, died 16 days post-surgery. There was no image evidence of liver cirrhosis or varicose veins at his initial evaluation. However, he had an episode of acute hepatitis during neoadjuvant concurrent chemoradiation therapy. The hepatitis was controlled before surgery. The Child score before surgery was B. Despite the high perioperative risk because of hepatitis, the patient urged us to do the surgery. During the laparoscopic portion, we saw that his liver was shrinking and that it had an uneven surface. Because the esophagectomy was finished, we were unable to discontinue the procedure. The operation was finished without any intraoperative problems, but the patient rapidly developed hepatic failure, a progressively elevated bilirubin level, and hemorrhage. Respiratory failure, anastomosis leakage, and sepsis soon developed as well.
The 3rd robotic arm permits self-controlled assistance.12 It is very useful for retraction and exposure and thus dramatically reduces the assistant’s work. Both for MIEs and for three-armed RAMIEs, 1 or even 2 experienced assistants are required for retraction, which ensures that the surgical table will be crowded. The assistant actually must be very attentive to prevent the robot arms from colliding with each other. With a four-armed RAMIME, we have never needed more than 1 assistant.
Technical Considerations
Avoid thermal injury
Sarkaria et al.12 described an ultrasonic-energy-instrument-related thermal injury. They illustrated 2 thermal-injury-related tracheobronchial fistulas and suggested that a unipolar-energy instrument replace the ultrasonic-energy instrument. In our experience, both bipolar- and unipolar-energy instruments can also cause local thermal injury. The ultrasonic-energy instrument itself will not cause more thermal injury. We adapted the rotation force to build a tension plane when doing dissections. By rotating the ultrasonic shears away from viable tissue, most thermal injuries were avoided. Because the freedom of the ultrasonic-energy instrument is limited, greater skill is required to use it well and safely.
Greater gastric curve visualization
Sarkaria et al.12 also described the difficulty of greater gastric curve visualization, a problem that caused several conversions to open surgery in their series. In the present study, however, this problem did not lead to any conversions, because we managed greater gastric curve visualization using the 3rd robotic arm retraction without a grasp. The double fenestrate grasp was routinely used on the 3rd robotic arm in this procedure. We grasped no tissue with it. Instead, we used its long instrument tip to retract the stomach in a parallel direction. Retraction was very useful in the body area; however, in the fundus area, it was not as useful because no downward retraction force can be applied without grasping. Thus, we managed the fundus area via the lesser curvature after the left gastric artery had been transected, which always worked. Therefore, we injured no stomachs because we grasped none. The circulation of the gastric conduit was always good. There is another critical trick for greater curve visualization. Do not place the camera port far above the umbilicus. The robotic camera cannot turn backward as it can in laparoscopy. In our experience, the umbilicus is the best site for the camera port.
Liver retraction
To manage the hiatal region, a good liver retraction is critical for a proper view. Traditionally, a liver retraction is done using a Nathanson liver retractor. A small puncture in the sub-xyphoid region is required to set up this instrument. However, the retractor is not necessary. We did the liver retraction using a simple handmade device composed of a straight needle, string, and a segment of a JP drain.15 The liver was punctured about 2 cm from the liver border and hung on the peritoneal wall. This afforded us an excellent view of the hiatal region. We never encountered complications with this procedure. Moreover, liver-border circulation appeared to the naked eye to be much better than when using the Nathanson retractor.
Gastric conduit formation
For the past decade, a growing number of studies have described the benefits of a narrow gastric conduit. Instead of the gastric pull-up, a narrow tube-like gastric conduit has less food retention and a lower regurgitation rate. In the USA, Luketich et al.16 reported a method of gastric conduit formation, and in Taiwan, Wang et al.17 reported a practical and easier approach: cutting off the lesser curvature. We used a modified version of the latter. When cutting off the lesser curvature, we left a narrow stump at the gastric cardiac portion. We then pulled up the remnant portion of the lesser curvature together with the gastric conduit to the cervical incision. Because both the hiatus and thoracic inlet were wide open after the dissection, there was no resistance when we pulled it up. It was effective and time-saving.
In summary, RAMIME with a four-arm platform is feasible. In comparison with McKeown MIE, the three-dimensional vision, camera stability, and the high degree of freedom together with the 3rd robotic arm suggest a promising future for RAMIME. Furthermore, because an esophagectomy takes a long time to complete, the comfortable sitting posture when doing RAMIME makes the procedure even more attractive to esophageal surgeons.
Conversely, the robot is a new surgical platform with some limitations because it requires a large workspace. It also takes some time to get used to the limitations of the robotic platform, e.g., limited contralateral arm maneuverability and collisions between robotic arms. With enough practice and understanding of the operating rules, a rational practice program that will remove most of the robot’s drawbacks can be developed.
Additional long-term cohort studies are necessary to adequately evaluate patient outcomes for operations using the robot. Multicenter studies with larger and randomized study populations should confirm the strengths and weaknesses of RAMIME. Initial experiences have revealed results that are at least comparable to those of MIE. With good understanding of the robotic concepts and a good robotic team, RAMIME is worth trying. It has the great advantage of replacing at least one assistant, and initial outcomes indicate that RAMIME is feasible.
CONFLICTS OF INTEREST: None declared.








