INTRODUCTION
Rhabdomyosarcomas (RMS) are the most common soft tissue sarcomas in childhood and account for approximately 3% to 8% of all malignancies in children and adolescents with an annual incidence of rate between 4 and 5 per million in children younger than 18-year-old. The genitourinary tract is the site of the primary tumor in the 10-26 % of these cases and occurs more often in bladder and pelvic organs.1
These tumors are to be considered highly malignant neoplasms and arise from skeletal muscle progenitor cells (pluripotential mesenchymal cells of connective tissue).2,3,4,5,6 RMS of the kidney is extremely rare.
We report one case of the ERMS localized in renal pelvis in children, focusing on the role played by the imaging techniques and the difficulty of choosing therapy in this unusual site of origin of the tumor, compared with other pediatric cases reported in literature.
CASE REPORT
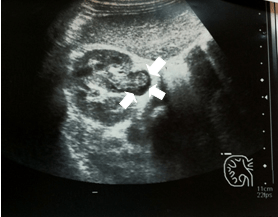
An 8-year-old boy was admitted to the emergency department of our hospital with a 15-day lasting recurrent abdominal pain, expecially located in the right side, and hematuria. No history of lumbar thrauma or stones was detected. The general physical examination was normal. Ultrasonography revealed the right ureteropyelectasy of the right kidney including a corpusculated and echogenic image that extends from renal pelvis toward inferior basin with no hypervascularization. This image was suspected for a clot (Figure 1). From this image we suspected the presence of a clot. It was always detected with the same features in the other scans performed in the following days. For a better image definition we performed an urography-magnetic resonance imaging (Uro-MRI).
Figure 1: Ultrasonography Revealed a Ureteropyelectasy of the Right Kidney including a Corpusculated and Echogenic Image, no Hypervascularization, that Extends on from Renal Pelvis toward Inferior Basin
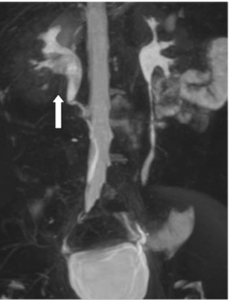
The image confirmed the lesion without vascularization in the right renal pelvis, while the T2 phase showed a mass, with a diameter of 15 mm, arising from the right inferior calices, in contiguity with the pelvic lesion, with intense enhancement after the administration of a contrast agent (Prohance 0.1 ml/kg), especially at the venous phase (Figures 2, 3 and 4). The radiologic assessment excluded metastases. Therefore, a percutaneous ultrasound-guided renal biopsy was performed. Three biopsies revealed a botryoid embryonal rhabdomyosarcoma.
Figure 2: Uro-MRI (T2) Showed a Rounded Material With Defined Margin (15 mm) in the Inferior Calices
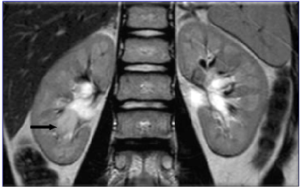
Figure 3: Uro-MRI (T1-Venous Phase) Showed the Lesion in Inferior Calices with Intense Enhancement
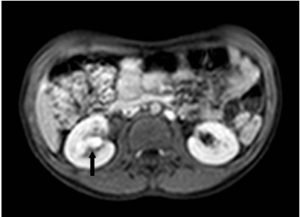
Figure 4: Uro-MRI (MIP) Showed an Amputation of the Inferior Calices in Right Renal with a Filling Defect in the Right Renal Pelvis
The patient subsequently underwent an open nephroureterectomy (Figure 5) with a fat layer sampling, bone marrow biopsy, and placement of a central venous catheter. We decided to leave intact the bladder wall (and not to perform the ureterectomy until the bladder) because the tumor was completely located inside the kidney. Finally the anatomical pathological examination showed the actual size of the tumor: 5×2.5 cm (Figure 5). Histologically it presented undifferential oval cells with poor cytoplasm, hyperchromatic nucleus and irregular necleolus. In minor components, there were elongated or round cells with eosinophilic cytoplasm, typical for the rhabdomyosarcoma. It was associated with inflammatory component. The mitotic activity was elevated. Immunostains were positive for Desmin, Myogenin, Actin, whereas negative for Cytokeratin, S100 proteins. The ureter, the fat layer and the Gerota’s band were free from disease. No evidence of hilar node involvement was detected. The post-operative course was regular. The infant underwent a chemotherapy according to EpSSG RMS 2005 (standard risk-subgroup B): Ifosfamide, Vincristin, Actinomicyn (13 cycles of chemotherapy). No recurrence or metastases were found after 5 years follow-up.
Figure 5: The Embryonal RMS just been Removed
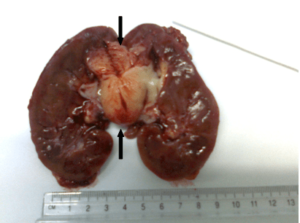
The Tumor Size was 5×2.5 cm. The Epicentre was in the Pyelic Region with a Secondary Involvement of the Inferior Calices.
DISCUSSION
This is a rare case in pediatric population. This tumor can easly mimic other tumors of kidney in pediatric age group, as nephroblastoma or rabdoid tumor.7,8 Therefore, the RMS with renal localization in children, should be considered in the differential diagnosis with other renal tumor of childhood.
The ultrasonography is frequently used as radiological examination of children who were affected by tumors of the soft tissues, since it is easily executable and well shows for visualization of the vascularization of a tumor.9 In our case, the ultrasonography examination provided a precise topographic description of the lesion that extends from renal pelvis toward inferior basin, and offered the initial suspicion of a tumor. But only the Uro-MRI revealed that the tumor was divided into two components: the first one protruding into the pelvis without specific radiological features (non-vascularised, located at the right renal pelvis simulating a clot) and the second one (approximately 15 mm long) arising from the papillary region and extended to the inferior calices with radiological characteristics typical of cancer (intense enhancement in the venous phase).
So our case confirms that the Uro-MRI is the gold standard imaging modality in RMS, although the imaging characteristics of RMS are relatively non-specific.10,11 Interestingly, some authors report that distinct renal tumors can correlate with imaging findings mimicking each other (the “claw sign”).12
The RMS is a complex childhood malignancy with ubiquitary anatomic sites of presentation and varying histological types, each presentation with peculiar patterns of growth, clinical behavior and prognosis.13 This complexity is reflected in the radiological assessment that remains highly challenging. Prognosis is dependent on anatomic primary tumor site, age, completeness of resection, presence and number of metastatic sites, histology and biology of the tumor cells.
No specific treatment guidelines for renal RMS have been established. In our case the pathology, the postsurgical stage, the node stage, the size and age were favourable, but the rare site didn’t ensure absolute safety for low risk. For this reason, the case was classified as a standard risk, subgroups B (EpSSG RMS 2005) and the infant underwent a chemotherapy with Ifosfamide, Vincristin, Actinomicyn (IVA). Few cases are described in the literature.12,14,15
The most important statistic revealed 6 cases of primary renal embryonal rhabdomyosarcoma (ERMS).16 Senga et al17 reported 15 cases of RMS of the kidney in Japanese literature. Small numbers of patients make it unwise to draw broad conclusions.
In our experience it was very important to perform an initial mass biopsy for a certain typing. Atypical localization of rhabdomyosarcoma should be considered as an unfavorable form. In addition, complete tumor removal and biopsy of contiguous lymph nodes are required in order to establish a radiotherapeutic treatment. The treatment of RMS requires a multidisciplinary approach, in which pediatric oncologists, radiologists, pediatric surgeons and pathologists altogether play a vital role.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
The authors have received written informed consent from the parent of the patient.










