INTRODUCTION
Taeniidae is the largest family of flatworms (tapeworms) representing the order cyclophyllidae. It comprises numerous tapeworms with medical and veterinary importance.1 Tapeworms 4154(Cestode) of the family Taeniidae are transmitted from the definitive host such as carnivores to the intermediate hosts in1uding herbivores or omnivores and human beings via oral-fecal cycle.2 Taenia saginata and Taenia solium are the two taeniids of greatest economic and medical importance, causing bovine and porcine cysticercosis and taeniasis in humans.3 Echinococcosis, also called hydatidosis, is a zoonosis and in humans, it occurs as a result of infection by the larval (metacestode) stages of taeniid cestodes of the genus Echinococcus. It is characterized by long-term growth of Metacestode (larval) stages (hydatid cysts) in internal organs (mainly the liver and lungs) of intermediate host animals.4
The diagnosis of taeniasis is based on the detection of eggs by microscopic observation of fecal samples. This technique lacks both sensitivity and specificity since the eggs of most members of the family Taeniidae are morphologically indistinguishable.5 Similarly, differentiation of T. solium and T. saginata is based on the morphological characteristic of the scolex or gravid proglottids. Recovery of scolices after treatment is uncommon for T. solium and in many cases, both the scolex and proglottids can be recovered only after special treatment.6 Detection of T.solium coproantigen by the enzyme-linked immunosorbent assay (ELISA) technique is used. The method is more sensitive than microscopy but cross-reacts with T. saginata.7
Molecular approaches8 have attempted DNA differential diagnosis of taeniasis and cysticercosis by Multiplex PCR. Also, the presence of Metacestodes in animals is detecting by Multiplex PCR with cytochrome-c oxidase subunit 1 gene yield evident differential products unique for T. saginata, T. asiatica, and T. solium reviewed by.9
Molecular methods have also been used to determine species or genotypes of taeniids using ‘pure’ parasite DNA obtained from adult worms or Metacestodes from intermediate hosts.10 However, the potential of these approaches to identify or differentiate among species of taeniid eggs in faecal or environmental samples had not been evaluated. Several polymerase chain reaction (PCR) assays have been developed for the specific identification of E. multilocularis from such samples.11 The specific identification of E. granulosus eggs using monoclonal antibodies has been described but this method has not been utilized in further epidemiological studies.10 Therefore, the aim is to explore the developments of the molecular diagnostic test for cestodes and metacestodes of veterinary importance.
CESTODES AND METACESTODES OF VETERINARY IMPORTANCE
Cestodes are a large diverse group of platyhelminths that share two common features: as adults, they have an elongate body, and they lack an alimentary canal. Thus, adult tapeworms is almost invariably found in the definitive host intestine where they absorb nutrients directly across their tegument.12
Taenia Solium/Cysticercus Cellulosae
T. solium causes Cysticercosis in pigs and as for T. saginata, humans are the obligate definitive host. Unlike T. saginata, the eggs from the adult T. solium that are present in the faeces of a tapeworm carrier can infect not only the natural animal intermediate host (pigs) but are infective the person who might accidentally ingest the eggs. In humans the cysticerci may encyst in the brain, causing neurological disease.13
Taenia Saginata/Cysticercus Bovis
Taeniasis is a cestode (tapeworm) infection of humans, with the adult phase of the worm residing in the intestine. Man is the definitive host of the cestode Taenia saginata (beef tapeworm). The intermediate host of the larval form of T. saginata is mainly domestic cattle. Beef tapeworm is transmitted when terminal segment (gravid proglottids), which each contain up to 100,000 eggs of T. saginata are detached from the segment chain (strobila), one by one and are passed in the feces of an infected person and the excreta is deposited in pastures or grazing area where the eggs are ingested by cattle.14 Cysticercus Bovis is caused by the metacestode stage of Taenia saginata, a zoonotic tapeworm of cattle and humans. Adult tapeworm develops in humans who consume undercooked beef infected with viable Metacestodes.15
Taenia Hydatigena/Cysticercus Tenuicollis
Cysticercus tenuicollis is the metacestode of canine tapeworm Taenia hydatigena, which has been reported in domestic and wild ruminants, pigs, monkeys.16 Metacestodes are found attached to the omentum, mesentery, and occasionally on the liver surface, however, unusual location of C.tenuicollis have been described as lungs, kidneys, brain, ovaries, uterine tubes, uterus, cervix, and vagina. An aberrant location of C.tenuicollis vesicle inside the chorioallantoic membrane of a goat foetus was reported.17 Pathogenicity of adult parasites is not high for the definitive hosts. However, a large number of developing cysticerci migrate contemporaneously in the liver of intermediate hosts, producing “hepatitis cysticercosa” a condition whose gross pathology resembles acute fasciolosis and which is often fatal.18
Echinococcus/Hydatid Cyst
Cystic Echinococcosis or cystic hydatidosis is a chronic helminthic zoonotic disease with a cosmopolitan distribution19 and is especially prevalent in the sheep-raising countries. The causative organism, the dog tapeworm Echinococcus granulosus is transmitted cyclically between canines and numerous herbivorous livestock animals, which can serve as intermediate hosts. In herbivorous animals and in people who become infected by accidentally ingesting E. granulosus ova, the cystic larval form (Hydatid Cyst) develops and can cause serious morbidity.20 Likewise, the disease often has severe consequences for health.
DIAGNOSTIC TECHNIQUES FOR CESTODES AND METACESTODES
Bovine and Porcine Cysticercosis
The most widely used approach for DNA identification of Taenia taxa has been to target the nucleotide sequences of fragments of selected genes using pairs of concerned PCR primers..15 The variable segment between the primers is PCR amplified for a particular Taenia sample and then directly sequenced. The mt cox1, nad1, cob, and 12S rDNA genes, and nuclear 28S rDNA and ITS1/ITS2 rDNA have proven particularly valuable markers amenable to this approach.26 Given that different DNA markers have different rates of evolution and conserved sites, the results obtained from analyzing the same samples may be inconsistent. The mitochondrial cytochrome c oxidase subunit 2(cox2) gene has been widely used in studies of evolution and genetic diversity in many species.2
Conventional Polymerase Chain Reaction
Using primer designed to hybridize with the region of the 18S and 28S ribosomal gene of DNA taken from T. solium (eggs, cysts, immature and mature worms) and T. saginata (eggs and mature worms).27 PCR detection of swine cysticercosis genomic DNA extracted from swine sera using different protocols showed higher specificity (100%) with no cross-reaction to trichinellosis and toxoplasmosis. sensitivity was lower Cox1 PCR (23%), T3/T4PCR(32%), and Nested PCR (64%) then ELISA-based detection of antibody from serum in the same study.28
Restriction Enzyme Analysis
DNA based differentiation of T. saginata and T. solium has been described and includes the use of probes.29 PCR with species-specific primers and PCR followed by restriction enzyme analysis (PCRREA).30 However, these methods require pure parasite DNA, which means that the DNA has to be extracted from a single proglottid and adequately cleaned, because these primers may amplify any eukaryotic DNA, causing cross-amplification. A few reports must describe the use of DNA-based techniques to differentiate T. solium from T. saginata from the fecal sample31 but they still lack sensitivity. Nonetheless, nowadays studies describe a; copro-PCR; for the simultaneous detection of the human tapeworm T. solium and T. saginata. Nkouawa32 reported stool PCR and loop-mediated isothermal amplification (LAMP) can distinguish between T. saginata and T. solium.
Real-Time Polymerase Chain Reaction
Newly developed a Real-time PCR to allow the sensitive and specific diagnosis of targets the COI gene of T. saginata by using TsagF oligonucleotide primers the technique yield 131 bp. When the results were compared with the reference A multiplex PCR the assay was less sensitive but offered the advantages of faster turnaround time and reduced contamination risk.15
Multiplex Polymerase Chain Reaction
As regards the identification of taeniid cestode parasites, multiplex PCR using taeniid species-specific and Taenia solium genotype specific-primers yielded differential products unique for T. saginata, T. asiatica, and Asian and American/African genotypes of T. solium with the molecular size of 827,269,984, and 720 bp, respectively (Figure 1).33
Figure 1. Multiplex PCR Amplification Products for Differential Detection of T. saginata, T. solium, and E. granulossus34
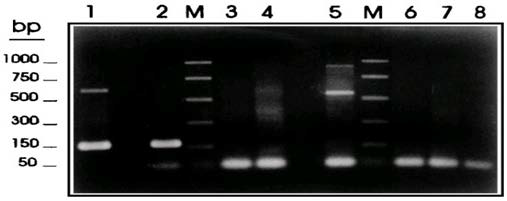
Gonzalez et al34 used Multiplex PCR for differential detection of T. saginata, T. solium, and E. granulossus based on the PTs7S35F1, PTs7S35F2, and PTs7S35R1 primers derived from the T. saginata genomic sequence HDP2. Samples of genomic DNA (1ng) from T. saginata (lane 1), T. solium (lane 2), T.taeniformis B (lane 3), T. taeniformis M (lane 4), E. granulosus (lane 8). Promega PCR molecular markers were used (lanes M).
Samples of genomic DNA (1 ng) from T. saginata (lane 1), T. solium (lane 2), T.taeniformis B (lane3), T.taeniformis M (lane4), E.granulosus (lane 5), a calf (lane 6), and a human (lane 7) were amplified by the multiplex PCR based on the PTs7S35F1, PTs7S35F2, and Ts7S35R1 primers derived from the T.saginata genomic sequence HDP2. A negative control without DNA was also included (lane 8). The reactions were carried out as described in Materials and Methods. The amplification products were fractionated on a 2% agarose gel and were stained with ethidium bromide. Promega PCR molecular markers were used (lanes M).
Nested-Polymerase Chain Reaction
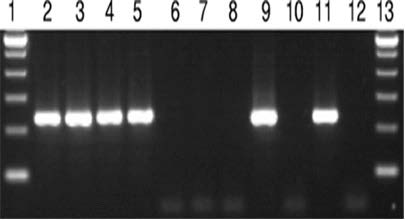
Mayta et al35 reported Tso31 nested-PCR amplification using DNA extracted from different sources. Electrophoresis was performed using 5 micros (1) of the amplification product. Lane 1 and 13, 100-bp ladders; lane 2 to 8, DNA from a contaminated sample with 100, 50, 20, 10, 5, 2, and 1 T.saginata proglottid; lane 9, DNA from a T. solium proglottid; lane 10, DNA from a T. saginata proglottid; lane 11, DNA from a T. solium-positive stool sample; lane 12, DNA from a T. saginata-positive stool sample (Figure 2).
Figure 2. Nested PCR Amplification Products using DNA Extracted from Different Sources35
Restriction Fragent Length Polymorphism
Molecular approaches include restriction fragment length polymorphism (RFLP) analysis, PCR-linked RFLP analysis (PCR-RFLP), and direct comparison of PCR-amplified DNA sequences.36 Sequence data of mitochondrial NADH dehydrogenase subunit 1 (mt-ND 1) and cytochrome c oxidase subunit 1 (mtCO1) genes of genus Taenia (T. taeniaeformis, T. hydatigena, T. pisiformis, T. ovis, T. multiceps, T. solium, and the Asian Taenia) is available on gene bank.37
Geysen et al38 performed PCR-RFLP by amplification of two sequential rounds. In the first round, primers ITMTnR and TaenF were used to amplify 846 nucleotides. A second round was performed using ITM TnR and nTAE to amplify a sequence of 766 nucleotides. lanes 4, 6 to 18 and 20 to 26 display a band of approximately 800 bp and are positive PCR results. Lane 3 and 5 are PCR-negative sample results. Lane 27 and 29 are negative control samples (Milli instead of extracted DNA) and lanes 28 and 30 are positive control samples (T. crassiceps DNA). Lanes 2 and 19 are DNA size markers (Figure 3).
Figure 3. PCA Results of T.saginata Lesions. Amplification was Performed in Two Sequential Rounds38
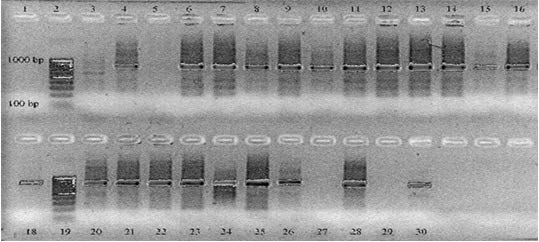
In the first round, primers ITMTnR (5CTCAATAATCGAGGGTGACGG3) and Taen F (5GTTTGCCACCTCGATGTTGACT) were used to amplify 846 nucleotides. A second round was performed using ITMT n R and TAE (5CGTGAGCCAGGTCGGTTCTTAT3) to amplify a sequence of 766 nucleotides. Lane s 4, 6 to 18, and 20 to 26 display a band of approximately 800 bp and are positive PCR results. Lanes 3 and 5 are PCR-negative sample results. Lanes 27 and 29 are negative control samples (MilliQ instead of extracted DNA) and lanes 28 and 30 are positive control samples (T. crassiceps DNA). Lanes 2 and 19 are DNA size markers.
Echinococcus/Hydatid Cyst
Conventional polymerase chain reaction:
Genetic variation in Echinococcus has been investigated using sequences from both the nuclear and mitochondrial genomes. The advent of the polymerase chain reaction (PCR) has provided a highly sensitive approach that is now widely used for Echinococcus identification purposes.39 Loop-mediated isothermal amplification assay primers (F3 and B3) for amplification mitochondrial NADH-1 gene of EG-complex Hydatid Cyst cattle strain of genotype 5(G5), was used as a target for using conventional PCR. Visualization of the 200-bp specific DNA PCR products on ethidium bromide-stained agarose gels.40
Conventional PCR targeting the E.granulosus specific DNA sequences of the NADH1 gene was performed on all serum, urine, and Hydatid Cyst fluid samples. The resultant 450 bp amplification products were observed on a 1.2% agarose gel following electrophoresis.41 Boufana et al42 described conventional PCR assay based on the amplification of a fragment within the NADH dehydrogenase subunit 1 (ND1) mitochondrial gene were optimized for the detection of Echinococcushi quicus, Echinococcus granulosus, G1, and Echinococcus multiloclaris DNA-derived from parasite tissue or canid fecal samples.
Real-time polymerase chain reaction:
A real-time PCR for the differentiation of the G1 and G2/G3 genotype of Echinococcus granulosus has been developed it has been suggested to offer several advantages over conventional PCR for the detection of parasitic infections, including increased sensitivity and specificity, reduced reaction time, and a quantitative estimate of the amount of bDNA in the sample (which May relate to both the infectiousness of the sample and the possible burden of infection.43
Restriction fragment length polymorphism:
Azab et al44 developed restriction fragment length polymorphism (RFLP) analysis using conventional southern blotting. without loss of resolution or accuracy, by linking RFLP analysis with PCR targeting the nuclear ribosomal DNA (rDNA) internal transcribed spacer 1(ITS-1) region. The random amplified polymorphic (RAPD)-PCR(RAPDPCR) has also been used under careful conditions for distinguishing the four recognized Echinococcus species and genetically distinct forms of E. granulosus.
Nested Polymerase Chain Reaction
Complete sequences of the mitochondrial (Mt) genomes of the horse and sheep strain of E. granulosus and E. multilocularis, and the availability of mt DNA sequences for several other E. granulosus genotypes, has provided additional genetic information that can be used for more in-depth strain characterization and taxonomic studies of these parasites. While Nested PCR on mitochondrial 12S rRNA gene shows 100% specificity when it was tested against E. multilocularis and E. granulosus isolates.45
Nested PCR products from 60 positive fecal samples were randomly selected and underwent hybridization with the specific probe E. multi. 1. with all samples a hybridization signal was obtained. E. multilocularis metacestodes yielded the same characteristic band of 250 bp (Figure 4).
Figure 4. Nested PCR Amplification of DNA from Nine Positive Fox Fecal Samples46
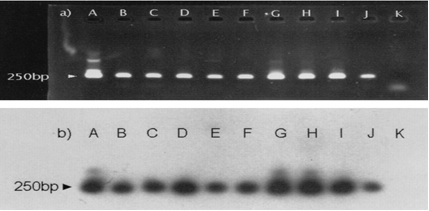
Ethidium bromide staining of 10 l of PCR products after 1.5% agarose gel electrophoresis showed the specific 250 bp band (a). The reaction products were analyzed by Southern transfer and hybridized with internal oligonucleotide E. multi. 1. labeled at the 5 end with digoxigenin(b). Lanes A, positive control; lanes B, C, D, E, F, G, H, I, and J, positive fox fecal samples; lanes K, negative control.46
Multiplex Polymerase Chain Reaction
Stieger et al47 introduced Multiplex PCR amplify partial sequences of the mitochondrial genes for NADH dehydrogenase subunit 1 (nad 1) for detection of E. multilocularis, and the small subunit of ribosomal RNA (rRNA) for detection of E. granulosus and Taenia spp amplification products were visualized by 2%(W/V) agarose gel electrophoresis, and the 395,117, and 267 bp expected fragments were examined for the presence of E. multilocularis, E. granulosus, and Taenia spp, respectively. DNA isolation from Fox excrement was performed according to a novel procedure involving lysis in KOH, phenol-chloroform extraction, and purification steps on a matrix (prep-A-Gene). The target sequence for amplification was the E. multilocularis U1 snRNA gene.48
Beiromvand et al48 reported by sequencing of E. multilocularis positive samples. Multiplex PCR showed 30 of 85 captured specimens (35.3%) to be infected with E. multilocularis and 14 (16.5%) infected with Taenia spp. by amplification of 395 bp fragment of nad1 and 267 bp fragment of rRNA, respectively (Figure 5).
Figure 5. Multiplex PCR Amplification of Mitochondrial genes of E.multilocularis and Taenia spp48
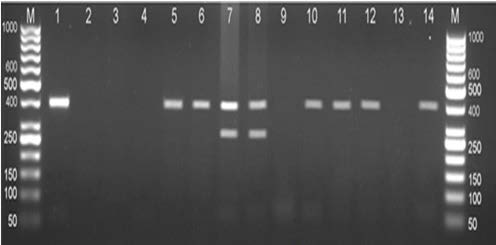
DNA extracted from the liver of Small mammals. Lane M, 50 bp DNA ladder (Fermentas; Cat No SM0373); Lane 1, positive control, a standard DNA of E. multilocularis (395 bp); Lane 2, negative control; Lane 5, 6, 10-12 E. multiloclaris; Lane 7 and 8, mixed infection of E. multilocularis (395 bp) and Taenia spp. (267 bp); Lane 3,4,9, and 13 negative samples.
Single Strand Conformation Polymorphism
Single-strand conformation polymorphism (SSCP) has been used to rapidly a large number of Echinococcus isolates. Another useful mutation scanning method is dideoxy fingerprinting (ddF), which is a hybrid between SSCP and conventional dideoxy sequencing. The technique has been used reproducibly for the direct display of sequence variation in the Cox1 gene to type and differentiate all of the Echinococcus genotypes examined.49 BartholomeiSantos et al50 isolate and characterize microsatellites using eight different oligonucleotides containing particular repeats as probes. Microsatellite DNA analyses have identified polymorphisms within the E. multilocularis endemic region.
CONCLUSION
In conclusion, the infection of cestode and metacestode of veterinary importance such as Echinococcosis and Cysticercosis contribute to a high-level of human and livestock production losses and morbidity. That is, Taenia saginata, Taenia solium, and Echinococcus cause production loss in bovine, sheep, goat, and pig respectively. As compared to other diagnostic techniques most molecular methods have higher sensitivity and specificity but due to the relatively higher cost, few are commercially available. Most of the molecular diagnostic tests developed to date are generally applicable for laboratory research purposes. The developments in the genomic and proteomic analysis should be used for further understanding of parasite-animal host interaction to find additional targets for diagnosis.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.










