INTRODUCTION
Leptospirosis has a global impact on human health and is considered to be burdening the world economy. More than 60,000 people die due to leptospirosis annually and nearly one million are reported to be affected.1 During the past several years, a large number of leptospirosis outbreaks have shook many parts of the world. In addition to Indian sub-continent Oceania, Caribbean and Latin America are considered endemic to leptospirosis.2
Leptospirosis, classified as a neglected tropical disease, is caused by a pathogenic spirochete bacterium of the genus Leptospira. 3 Besides leptospira are classified in to two leptospira interrogans are pathogenic species that cause Leptospirosis whereas L. biflexa is non-pathogenic.4 Although leptospirosis is one of the world most widely spread bacterial disease caused by the genes, which are classified as a direct anthropozoonosis, it affects humans and a wide range of animals. Leptospirosis traditionally has to be considered as rural based diseases however, urban epidemic associated with severe form of the disease is also reported to occur annually resulting in significant mortality.5
Pathogenic Leptospira lives in the kidneys of natural hosts predominantly mammals, and are excreted with the urine into the environment where they survive for up to several months depending on favorable conditions. Like, infection occurs by contact with infected animals, their urine or via urine contaminated environment (mostly mud and water). The infection route is through open skin and mucous membranes. Leptospirosis is commonly diagnosed in several animal species a large number of sylvatic and domestic animals serve as reservoir of Leptospira. Human infections caused by L. interrogans of which there are over 200 known Pathogenic serovars and several modes of transmission are involved in leptospirosis.6
Human infection is caused by recreational exposure to water contaminated with Leptospira and therefore humans are considered to be accidental hosts whereas animals are considered reservoir hosts of Leptospira, a large number of clinical manifestations are associated with leptospirosis. The diagnosis of leptospirosis is based on the availability of the sample and the temporal stage of the disease. Laboratory tests used for the detection of leptospira are microscope evaluation, culture, molecular methods, serology and anima inoculation.7
Treatments of Leptospirosis by antibiotics such as tetracycline, penicillin, ampicillin, doxycycline, streptomycin and erythromycin.8 The risk of infection occurring due to interaction with contaminated environments, infected wild animals as well as with synanthropic animals and rodents,9 control measures of leptospirosis are aimed at limiting the occurrence of clinical disease based on integrated actions in clinical disease based on integrated actions in several links of the transmission chain.10 Therefore, the objectives of this paper were to review on Leptospirosis and its Public Health Significance that affects Domestic Animals and Humans.
LITERATURE REVIEW
Etiology
Leptospirosis is caused by pathogenic spirochetes of genus Leptospira occurring in almost all the mammalian species,11 Leptospirae is unique among spirochetes like Leptospirosis is caused by spirochete’s from the genus Leptospira currently contains 20 species containing nine pathogenic, six saprophytic, and five intermediate species.12 Like Leptospires are mobile, their bodies are small diameter requiring the use of dark field microscopy or phase contrast for observation.
These bacteria are aerobic, do not resist drought or hypertonicity, however, they support alkalization to pH 7.8. Leptospires are thin, obligate aerobe, fine spiral shaped organisms with hooked ends having two or more axial filaments that are responsible for the motion of the spirochete, and visualized under dark field microscopy, the epidemiology of most serovars is poorly studied but certain serovars have been linked strongly to specific animal reservoirs6 some of the serotypes are present globally, while others are confined to certain place.
Leptospirosis is an infectious disease caused by L. interrogans complex, which has over 20 serogroups and more than 200 serovars. Dog, and pigs act as carriers for several months (temporary carriers) while rodent usually remain carrier throughout their life (permanent carrier). Rodents are therefore considered as the major reservoir of infection. Leptospires are excreted in the urine of the animals and they affect man when he is exposed to urine of infected animals, directly or indirectly,13 some of the serotypes are present globally, while others are confined to certain areas. Commonly known serovars are L. interrogans serovars pomona (swine), L. interrogans Bratislava (swine), L. interrogans Canicola (dogs), L. interrogans hardiso (cattle), and Icterohemorrhagiae and L. graphityphosa (rats).14
The bacteria are highly motility thin, flexible and filamentous, made up of fine spirals with hook-shaped ends.15 It is 0.1 μm wide and 6-20 μm long. In tissue and inside phagocytes, the organism will assume a spherical or granular look. Their narrow helical type permits Leptospira to burrow into a tissue. Leptospira have two periplasmic flagella, one attached sub terminally at each end that extends toward the cell center without overlapping, although the flagella lie inside the spirochaete outer membrane, they are integral to cell form and motility,16 the organism is sensitive to common disinfectants and antiseptic, and easily killed at 60 °C in 10 seconds. The case fatality range from three to over 50% (Figure 1).17
Figure 1. High-Resolution Scanning Electron Micrograph of Leptospira Interrogans Serovar Introhemorrhagiae
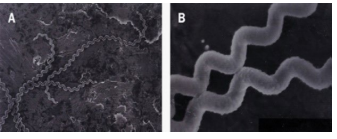
(A) Note characteristic hooked ends
(B) At high magnification the surface of the spirochete seems ruffled and beaded
The Leptospira genome has two circular chromosomes and the genome is larger compared with the genomes of the other spirochetes. This indicates that Leptospira species can live in a diverse environment like animal hosts and freely in the environment.18
They grow in simple media enriched with vitamins B2 and B12, the media used for isolation and cultivation of Leptospira are liquid or solid enriched with rabbit serum or bovine albumin. Fletcher’s semisolid medium, Korthof ’s liquid medium and Ellinghausen-McCullough-Johnson-Harris medium are the most commonly used media. On the other hand, no growth occurs on blood agar and other routines and some produce urease.19 Leptospires are motile and aerobic, cannot survive dry environment, heat, acids and basics disinfectants but can sustain alkali pH up to pH 7.8.20 Although the bacteria can only be visualized using a dark field or phase contrast microscope they are gram negative in nature, gram’s staining is not done for visualization of the bacteria. Like silver impregnation, staining, immune peroxidase staining or immunofluorescence should be done to observe these bacteria.21
Leptospirosis is aerobic in nature, the bacteria can be cultured media enriched with vitamins like B1 and B12, long-chain fatty acids, and ammonium salts at 28-30 °C. Of course fatty acids are the sole source of carbon and are utilized by β-oxidation.22
EPIDEMIOLOGY OF LEPTOSPIROSIS
Leptospirosis has a complex epidemiology as many animals serve as reservoir of infections, widely distributed geographically and occurs mostly in tropical, subtropical and temperate zones.23 Disease is recorded in both sexes with predominance in young adult males, and in urban and rural settings, it is an important occupational zoonosis of agriculture workers, sewer workers, mine workers, slaughterhouse employees, butchers, dairy farmers, veterinarians, animal handlers, kennel attendants, sanitary workers, construction workers, military personnel’s and fishermen.6 To occupationally exposed teams, urban slum dwellers in areas with poor sanitation are communities at significantly high-risk.24 Infection-related to outdoor recreational exposure, international travel, especially to endemic areas in the tropics and flooding is increasing.
Most communities in such areas collect water from natural water bodies such as rivers, streams, or underground aquifers, and then store this water for long periods of time are reservoir. It is globally being recorded as an epidemic threat in developing countries in and around the rainy season. In urban setting, flooding of roads during heavy rains expose the persons to Leptospira infection. However, in rural areas, farmers working in rice fields are at high-risk of acquiring infection.25
Host
All mammals seem to be prone to at least one of Leptospira. The principal reservoir hosts for most Leptospira serovars are wild mammals, specifically rodents. Cattle, sheep, goats, dogs and pigs are reservoir hosts among domestic animals and they act as a carrier for several months while rodents usual remain carriers throughout their life. Thus, rodents are considered the main reservoir of infection,26 the particular reservoir hosts differ with the serovar and the geographic region (Table 1). Leptospirosis in reservoir hosts is more probable to be asymptomatic, mild or chronic.27 Most cases of leptospirosis are asymptomatic or mild. The overall case fatality rate is 1-5%. The mortality varies with the form and is higher in the elderly. The icteric form is rarely fatal. The icteric form, which occurs in 5-10% of all patients, has an overall mortality rate of 5-15% and a 54% case fatality rate in severe cases with myocardial involvement. Most patients with kidney failure, hepatic disease or anterior uveitis eventually recover with full kidney or liver functions and vision.28
| Table 1. Maintenance and Incidental Hosts for Important Serovars of L. interrogans |
|
Serovar
|
Maintenance Host
|
Incidental Host
|
| L. Bratislava |
Pig |
Horse, Dog |
| L. Canicola |
Dog |
Pig, Cattle |
| L. graphityphosa |
Rodent |
Cattle, Pig, Horse, Dog |
| L. hardiso |
Cattle |
Human |
| L. interohemorrhagie |
Brown cat |
Domestic animals and human |
| L. Pomona |
Pig, Cattle |
Sheep, Horse, Dogs |
Source of Infection and Modes of Transmission
Leptospirosis is mostly transmitted by the urine of an infected animal and by contact with skin abrasion The type of habitats most likely to carry infective bacteria are moist and damp areas like riverbanks, muddy livestock rearing areas where there is a regular passage of wild or farm mammals.29 The disease is, seasonal in temperate climates and year-round in tropical climates and directly with the amount of rainfalls, leptospirosis is reported to be transmitted via the semen of the infected animals.30
Like humans are considered to be the accidental hosts as a result of direct or indirect contact with leptospirosis infected animals. Besides animal hosts of leptospirosis may be either carrier or reservoir hosts, the latter being the primary source of infection. Although, the presence of animal carrier is considered important in leptospirosis transmission but it can occur through various environmental sources, leptospirae being ubiquitous in nature are found everywhere but their primary habitat is renal tubules of carrier animals Leptospirosis has also been reported in various wild animals such as bats, possums, deer, mongoose and small insectivores.31
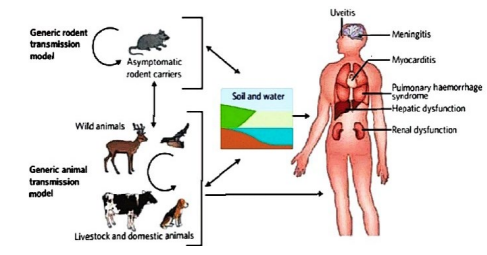
Direct transmission occurs through leptospires from tissues, body fluids or urine of acutely infected or asymptomatic carrier animals. Infection with leptospirae is maintained within a population of natural maintenance hosts by vertical and horizontal transmission, such population of natural animal host form the infection reservoir. Leptospirae can also be transmitted from human to human by sexual intercourse, trans-placental from infected mother to the fetus and though breast milk and vision problem and developmental delay in children (Figure 2).32
Figure 2. The Transmission Cycle of Leptospirosis
Risk Factors
Host and Management risk factors:
All age groups of animals can be affected by Leptospira infection. However, the leptospirosis is higher in young animals with higher morbidity. Although leptospirosis nearly occurs in all mammalian species, it occurs commonly in cattle, sheep, goats, dogs, horses and pigs, but the disease appears to be rare in cats.33 Certain management factors that pose risks of infection are diseased animals introduced into herds, common grazing with the infected one, access to contaminated water provides like streams, rivers, flood or drainage water and buying or loan of infected male animals for natural insemination.34
Pathogenic risk factors:
Virulent Leptospira resist the anti-bacteria action of complement and neutrophils in immune non-hosts, however, it is rapidly killed by either mechanism within the existing of particular epithelial and endothelial antibody,35 the ability of Leptospira to invade Vero cells and to reduce apoptosis in macrophages was correlated with virulence. Nevertheless, the organism must penetrate host epithelial and endothelial cell barriers for both hematogenous spread and localization in target organs, such as liver and kidney a cytotoxic glycolipoprotein fraction is showing to inhibit host ATPase with the activity ascribed to the presence of long chain fatty acid. Leptospira Pomona in cattle causes intravascular hemolysis due to hemolytic exotoxin.36
Public Health Implication of Leptospirosis
Risk factors in human:
Leptospirosis infection are occupationally risk groups that are exposed to animal reservoirs or contaminated environments, such as abattoir and sewage workers, salver workers, coalmines, plumbers, farmworkers, veterinarians, slaughter house employees, meat handlers, military personnel and employees within the fishing industry.37 Recreational activities that increase the risk of Leptospira infection are husbandry, and water sports like canoeing, swimming and white rafting residents of some urban areas.34 Men are more frequently diagnosed with leptospirosis compared with women and this has been traditionally attributed to the representation of men in high-risk occupational groups.38
PATHOGENESIS
The bacteria enter the body through small abrasions, mucosa, conjunctiva and genital tracts. It involves 33 some chemotactic interactions and a transmembrane passage. The bacteria settle down in the convoluted tubules of the kidney and thus keep on shedding the pathogen in urine. The period of shedding varies from a few weeks to many months. After the bacteria reach a higher concentration in blood and tissue, there is tissue damage due to endotoxins secreted by the pathogen, hemolysis is also secreted by the bacteria and leads to damage of blood cells. Endothelium gets damaged which leads to ischemia and other complications. The exact molecular basis of virulence is yet unknown; however, the humoral response has been observed to be active in the first week of infection leading to phagocytosis by macrophages and neutrophils.39
The primary lesion is damaged to the endothelium of small blood vessels leading to localized ischemia in organs, resulting in renal tubular necrosis, hepatocellular and pulmonary damage, meningitis, myositis and placentitis. Like the incubation period depends on infective dose, growth rate of organisms, their toxicity, and immunity’s, the correct characterization of leptospiral pathogenicity is strengthened by using a polyvalent analytical approach that minimizes uncertainties encountered from individual tests especially when phenotypic analysis does not strictly equate with genotypic speciation.40
The invasive capacity of leptospirae may be related to their pathogenicity because nonpathogenic leptospirae do not penetrate cells as deadly as pathogenic leptospirae. Damage to the endothelium of small blood vessels may result in ischemic damage to the renal parenchyma36 kidney are involvement in many animal special is chronic and results in elimination of a large number of leptospirae in the urine. This is the main source of contamination and infection of human and other mammals. Common symptoms also include malaise, fatigue, headaches, and mild depression also found in cervical and vaginal secretion, semen, breast milk, tears, urine, it is well known for causing disease through human contact with cat feces, and have atypical lymphocytes.
CLINICAL SIGN
The clinical findings in leptospirosis are both highly variable and non-specific, depending on both; host and pathogen factors that significant proportion of infections are; asymptomatic or subclinical, and when symptoms do occur, onset is typically 2 to 30-days after exposure, with average incubation time of 7 to 12-days.41,42 In humans the disease is characterized by variety of symptoms, including high fever, vomiting, jaundice (yellow skin and eyes), red eyes, headache, chills, muscle aches, abdominal pain, diarrhea, rash etc. unknown many of these symptoms can be mistaken for other diseases. some infected persons may have no symptoms at all.
The time between a person exposure to a contaminated source and becoming sick is 2-days to 4-weeks. The illness usually begins abruptly with fever and other symptoms. In animal the clinical signs of leptospirosis are often related to kidney this serovar is also maintained in farmed red deer and wapiti areas with a high disease incidence in adult cattle early signs such as fever and depression, a severe pulmonary form of leptospirosis. The clinical findings in leptospirosis are similar in each animal species and do not vary greatly with the specie of leptospirae except that infection with L. interohaemorragiae usually causes severe septicemia.
The clinical finding in the acute form of leptospirosis is maintained by septicemia, with high fever anorexia, petechiation of mucosa depression and acute hemolytic anemia with hemoglobin urea, jaundice and pallor of the mucosa. Milk production is markedly decreased and the secretion is red colored or contains blood clot and the udder is lop and soft. the subacute form of leptospirosis differs from the acute form only in degree. Fever is mild and hemoglobinuria is common but jaundice may or may not be present. the clinical findings in the chronic form of leptospirosis are mild and may be restricted to abortion. Abortion usually occurs during the last trimester of pregnancy.34
Infertility and milk drop occurs only in pregnant or lactating cows because Leptospira organisms require pregnant uterus and lactating mammary gland to proliferate. In humans, the disease is characterized by variety of symptoms, including high fever, vomiting, jaundice (yellow skin and eyes), red eyes, abdominal pain, diarrhea, rash many of these symptoms can be mistaken for other diseases. In addition some infected persons may have no symptoms.
Leptospirosis may Occur in Two Phases
Phase 1:
The first phase (acute or septic phase) ends after 3-7-days of illness with the appearance of antibodies against Leptospira and the disappearance of bacteria from the bloodstream. The Patient is asymptomatic for 3-4-days until the second phase begins with another episode of fever.
Phase 2:
The hallmark of the second phase is meningitis (inflammation of the membranes covering the brain) the illness lasts from a few days to 3-weeks or longer. Without treatment, recovery may take several months Majority of the cases of the leptospirosis are mild form. The classic form of severe leptospirosis is known as Weil’s disease, which is characterized by liver damage (causing jaundice), kidney failure, and bleeding. The disease affects brain also causing meningitis, encephalitis of brain tissue with same signs and symptoms; and lung affected as the most serious and life-threatening of all leptospirosis complications. The infection is often incorrectly diagnosed due to the non-specific.
DIAGNOSIS
Leptospirosis cannot be diagnosed alone on the basis of clinical manifestations but easy by laboratory diagnostic methods is required for correct diagnosis. Understanding the course of the disease, right specimen selection and the choice of diagnostic test are the essential factors that help in precise leptospirosis diagnosis, the clinical diagnosis of leptospirosis must be supported by a variety of laboratory tests such as microbiological, immunological, and molecular techniques.43
Like on infection the bacteria can be found in blood and cerebrospinal fluid (CSF) for the first 7 to 10-days and then moving to the kidneys. After 7 to 10-days the microorganism can be found in fresh urine because of the large range of symptoms, it can be incorrectly diagnosed as another disease, which is early diagnostic efforts, include isolation from blood or other clinical materials through culture of pathogenic leptospires.36
Diagnosis of leptospirosis is confirmed with tests such as enzyme-linked immunosorbent assay (ELISA)44 and polymerase chain reaction (PCR).45 Because clinical examination of leptospirosis are very non-specific and have significant confuse with a variety of other febrile illnesses, diagnostic testing because clinical manifestations of leptospirosis are very non-specific and have significant overlap with a variety of other febrile illnesses a combination of exposure history and symptoms should prompt confirmatory testing. However clinical suspicion alone may be enough to warrant empiric antibiotic treatment in many cases. In general, definitive diagnosis of leptospirosis can be made via either traditional microbiological methods (direct detection, culture) or (serology).21 Leptospira, like other spirochetes, stains poorly with traditional staining methods and is best visualized with dark field microscopy, however, sensitivity and specificity are both poor when examining clinical samples.46
Culture
Leptospira from patient samples is also challenging: the organisms typically take 1-2-weeks. to grow, but may take over a month, and special growth media is required, often necessitating advance notice to the lab. though specificity of culture is excellent sensitivity is very poor samples for culture should be collected prior to the administration of antibiotics.47 Samples should be stored and transported at ambient temperatures since low temperatures are detrimental to pathogenic leptospires.48 Leptospira spp. stain poorly with the Gram stain and are not observed by microscopy unless special stains or methods are employed. Silver staining or immunogold-silver staining is sometimes useful as an adjunct technique. Dark field microscopy can also be used to detect Leptospira. 21
Serology Based Methods
The most commonly used means of confirming a diagnosis of leptospirosis and also The “gold standard” is the microscopic agglutination test (MAT), in which acute and convalescent sera from a suspected case is mixed with a panel of live antigens from different serogroups of Leptospira organisms and examined for agglutination.42 While there is some variability amongst labs/references, most commonly, a single titer of 1:100 (range is 1:100 to 1:800), or a fourfold rise in titer between acute and convalescent sera, serologically confirms the diagnosis of leptospirosis.46
Microscopic Agglutination Test
The microscopic agglutination test (MAT) is still considered the gold standard test in diagnosing leptospirosis according to Centers for Disease Control and Prevention (CDC). Although Live Leptospira are to be cultured regularly and agglutination reaction is carried out to check the species and serovar of the pathogen, like it is a very laborious and expensive method and needs live bacterial antigen to test, that’s why this test is only conducted in specially designed labs.49,50
Several tests based on antigen-antibody agglutination are being used currently for the diagnosis of leptospirosis. It is considered to be serogroup/serovar specific when carried out on paired sera. However, microscopic agglutination test involves the treatment of patient serum sample containing leptospiral antibodies with live antigen. The agglutination between antibody and live antigen is observed under dark field microscope maximum agglutination has occurred.51
When no free leptospires are observed under the dark field microscope, maximum agglutination has occurred. The patient serum samples are continuously diluted till maximum agglutination has occurred and no free leptospirosis are available. The final serum dilution is the one at which 50% or more leptospires have undergone agglutination. Even though a number of studies have been carried out which exhibits the variable sensitivity and specificity of MAT in leptospirosis diagnosis. The sensitivity of MAT in diagnosing leptospirosis in the acute phase is considered very less.52
Enzyme-Linked Immune Sorbent Assay
The family Leptospiraceae consists of three genera via, Leptospira, Leptonema and Turneria. Leptospira consists of many pathogenic and non-pathogenic serovars characterized under it. Enzyme linked immune sorbent assay is considered to be the easy and simplest method used for leptospirosis diagnosis and also more sensitive than most of the serology based tests used for conventional diagnosis of leptospirosis.53
The ELISA test is much more accurate than other tests and has much advantage from point of view of laboratory practices, it has excellent diagnostic specificity and sensitivity, convenient technical feature including automation and can be used efficiently as screening test for large number of serum samples.54 Although, it is easy, safe and can detect IgM and IgG.55 It can be used in humans and animals.56 Elisa can be performed with minimal training and typically provides results in 2-4 hrs.50 It has been recommended for the rapid diagnosis of leptospirosis in endemic areas.57
TREATMENT
The primary aim of treatment of leptospirosis is to control the infection before irreparable damage to the liver and kidneys occurs. Treatment with antibiotics counseled as before long as doable when signs seem. The results of treatment are often disappointing because in most instances, animals are present for treatment only when the septicemia has subsided. The secondary aim of treatment is to regulate the leptospiruria of carrier animals and render them safe to stay within the group. Other antibiotics used to treat leptospirosis include tetracycline, penicillin, ampicillin, doxycycline, streptomycin and erythromycin.8
The efficacy of treatment may depend on the serovar Fluid therapy, blood transfusion and other supportive care may also be necessary. These supportive treatments depend on the animal and needed if the animal is severely affected and in shock, it will need fluid therapy. In beef herds, further abortions prevented by vaccination and treatment of all animals with antibiotics and in dairy cattle, only infected animals usually treated due to the potential loss of milk sales.58
CONTROL AND PREVENTION
Leptospirosis has been an underreported disease and there are a few reliable global incidence data as the disease is not detected in common laboratory tests. Like as the symptoms are very much similar to many other diseases that can confuse and the bacteria being destroyed by common antibiotics, Leptospirosis is very hard to detect in a population. Although the risk of leptospirosis can be greatly avoiding water contaminated with animal urine or contact with potentially infected animals. Like occupational workers should wear protective clothing or footwear. Any skin cuts should be covered with waterproof dressing while swimming in fresh water to protect against a range of infections. In pet dogs, vaccination against the bacteria protects the animal and the household, human immunization helps to provide a certain degree of protection against infection.59
The complete elimination of the disease seems unfeasible as many species of rodents serve as reservoir of infection. Hence, proper strategies for prevention of leptospirosis are based on awareness of leptospirosis epidemiology and transmission mechanisms. It is possible to prevent the disease by reducing exposure and implement protective measures, immunization, and pre- or post-exposure chemoprophylaxis. Housing construction that prevents entry of rodents from invading residential living areas and water sources greatly reduces risk of infection. Swimming in contaminated water, and walking barefoot in floodwater should be avoided.43
In animal reposition must be selected according to the non-reactivity of herds to leptospirosis. Available vaccines of leptospirosis for domestic animals can decrease the severity of the disease, although it cannot prevent the infection completely because the immunity is serovar-specific and vaccines protect only against serovars included in the immunogens.26
CONCLUSION AND RECOMMENDATION
Leptospirosis is the most common zoonosis of global relevance, caused by Leptospirosis interrogans, which are also the most pathogenic. Among the spirochetes, Leptospirae is unusual. It has an impact on both humans and animals. Although the bacteria are usually spread by infected animals’ urine and skin abrasions, the bacteria enter the body through tiny abrasions, mucosa, conjunctiva, and genital tracts in animals like rats and mice. Pathogenic Leptospira lives in the kidneys of natural hosts, mostly mammals, and is expelled with the urine into the environment, where it can persist for several months under favorable conditions. Antibiotics are effective treatments for leptospirosis, and current leptospirosis vaccinations for domestic animals can help to reduce the disease’s severity.
Therefore the scarcity of information regarding to leptospirosis in African countries, including Ethiopia. Leptospirosis is seriously contaminated urine is highly infectious for people and for susceptible animal species; therefore, contact with urine on mucous membranes or skin abrasions should be avoided. When handling infected animals or dealing with areas of contamination, protective gear should be worn such as gloves, eye protection and face masks. Although, easy to treat with most antibiotics, Leptospirosis is easy to diagnosis by microscopic, culture and serology.
Based on the above conclusion the following points are forwarded as recommendations:
• It is important to conduct applicable control techniques and increasing the public awareness about zoonotic transmission of leptospirosis is suggested.
• Besides, any study and control strength should be conducted by collaboration among human, animal and environmental health professions.
• More researches should be conducted on prevalence/incidence of leptospirosisin Ethiopia since very few findings are documented currently.
• Communities at risk should wear protective glove when exposed to animal reservoirs and contaminated environments.
• Government should involve in funding the research to be done on the status of leptospirosis in developing country including Ethiopia.
• Proper control measures and public awareness should be made in endemic areas.
ACKNOWLEDGMENT
In the name of ALLAH, the most Gracious and the most merciful Alhamdulillah, all praises to Allah. He blessed me with good health, intellectual efficiency, talented teachers and sympathetic friends, whose proper guidance, consistent encouragement and inspiration enabled me to start this seminar paper. I feel great pleasure and honor to express my heartiest gratitude and deep sense of obligation to my Advisor Migbaru K. (DVM, MSc in Biotechnology, Assistant Professor) for his keen interest, encouraging guidance, for his valuable advice, insight and guidance from the initiation to the completion of these seminar paper, his open-minded views, help and valuable suggestions for the successful beginning of this seminar paper.
We would like to thank Haramaya University College of Veterinary Medicine for this taken to write seminar paper.







