INTRODUCTION
Around 80% of crop insect pollination work is done by honeybees.1 However, a number of diseases brought on by diverse infections afflict this economically and ecologically significant insect. Fungi are among the common saprophytes of dead bees and combs in honeybees that cause sickness.2 The majority of fungi that are somehow introduced to the colonies are unable to grow within the beehives. Nevertheless, a number of fungi, including Ascosphaera apis (Maasen ex Claussen) L. S. Olive and Spiltoir, Aspergillus niger (van Tieghem), Aureobasidium pullulans (deBary and Löwenthal) Arnaud, Trichoderma lignorum (Tode) Harz, Mucor hiemalis (Wehmer), and Rhizopus, are considered to be honeybee fungal pathogens that are able to establish in the colony and cause problems.3
Most fungal infections in insects are brought on by the germination of spores, and the ability of the pathogen to infect honeybee larvae depends on its genetic potential to grow quickly, use host body components, produce cuticle-degrading enzymes to breach anatomical barriers, and resist host immune mechanisms.4 Of course, the pathogenesis of mycosis cannot be completely excluded from the battle for nourishment between the growing fungus and the diseased larval organs.5 Temperature, excessive humidity, environmental pollution, pesticide poisoning, parasite invasion, and predator attacks are the main physical, chemical, and biological stress factors that contribute to the formation of fungus in insect colonies.6 The same is true for the ideal method of disease transfer between colonies, colony-level reproduction.7
Numerous academics have highlighted that the honeybee system contains a vital pathogen transfer pathway for vertical pathogen transmission (pathogen transmission when the parental colony infects the daughter colony). It is evident that one cannot restrict such studies to individual bees or individual colonies when investigating the incidence and spread of disease pathogens in social insects. The spread of diseases between colonies must also be taken into account. However, very little research has focused on the real epidemiology of honeybee diseases, including both Apis cerana (Fabricius) and Apis mellifera (Linnaeus) species. In light of this, it should also be clear that honeybees, whether raised naturally or under management, should exhibit anti-pathogen virulence.8 Ascosphaera apis (Maassen ex Claussen) produces chalkbrood, an invasive mycosis that only affects bee brood in honeybees (Apis mellifera).9 It is a fungus that affects honeybee larvae and causes chalkbrood, an infectious illness that kills and mummifies sealed honeybee brood, weakening the colony.10 Although fatal to individual larvae, the disease does not usually destroy an entire bee colony. However, it can result in severe losses in bee populations and colony productivity, with documented 5-37% decreases in honey production.11,12 Honey bee colonies currently exist all over the world, and there are some signs that the prevalence of chalkbrood has grown recently.13 According to research by Aizen et al14 human activities associated with rising food demand have both direct and indirect impacts that may be at least partially to blame for this trend. Diseased brood is present throughout the brood-rearing season once the colony has been infected by Ascosphaera apis, but is most common in late spring when the brood nest is expanding quickly. Moreover, some disease symptoms, principles and techniques of detection, research methodologies and protocols, and treatments for chalkbrood have been documented.15
With an emphasis on pathogen biology, illness etiology, and control strategies, documented the historical knowledge and modern scientific findings in this review. This paper reviews and summarizes the historical background, pathogen biology and morphology, disease pathogenesis, insect immune system against challenges, major scientific findings so far, and suggested research directions in an effort to provide brief information on the latest status and developments regarding the chalkbrood disease, despite the fact that its distribution magnitude and economic thresholds have not been sufficiently surveyed, determined, and documented for the entire world.
REVIEW OF LITERATURE
Historical Incidence and Geographical Distribution
In global agriculture, honeybees play a critical role as commercial pollinators of crop monocultures, which depend on insect pollination.16 However, as the pollinator-dependent agricultural production is still increasing the number of managed honeybee colonies is declining which has become an international concern.17 This concern worldwide accompanied by various research works, over the past years, evidenced and suggested that causes are related to different pathogens.18
Even though its existence, as quoted by Samsinakova, was reported from Czechoslovakia in 1878, the chalkbrood fungus is believed to have been discovered by an American scholar, Dr. H. Priess, in 191118 in an infected comb sent to him from Hanover province in Germany
Beekeepers in this province called it “the trouble chalkbrood”, and this name has been generally adopted, although ‘Ascosphaerosis’ and ‘Pericystismycosis’ are also its names. Its notice by the beekeepers in the 1900s has been believed to be associated with the change of their system of beekeeping from fixed-to-movablecomb techniques. The disease has long been known in Germany, Scandinavia, Russia, and Great Britain.19
By 1977, chalkbrood had also been recognized as the most serious infectious bee disease in Norway. One of the earliest reports of the fungus outside of Europe was from New Zealand in 1957, and the disease was also observed in different areas of Argentina in 1978. After one decade, in 1988, high apiary infection levels were reported to occur throughout a large number of provinces in the country.20 Within the same continent, chalkbrood was also found to be widespread in Mexico before the year 1984. The disease has now been detected in most beekeeping areas of Chile, Central America, Japan, and the Philippines.21 The fungus was reported from Israel in 1984, and it was present at a very low-level up until 1990, when a substantial increase in the rate of infestation was detected.5
Even if the source of infestation in Turkey was assumed to be through the importation of contaminated bee wax from several countries between 1986 and 1988, surveys conducted in the spring of 1988 showed that chalkbrood was widespread in many honey-producing regions of the country.22 Although it has been thought that its appearance is much earlier, chalkbrood in the United States has been reported first from Utah in 1965, from California in 1968, and then in 1971 from Southcentral Nebraska. The latest was reported by a dead larvae sample sent to the U. S. bee disease investigation laboratory by a commercial beekeeper who had moved his colonies from Texas. From 1972 to 1975, the disease was reported to have spread to numerous parts of the country.23
At the same time, it was found to be prevalent in the mid-western and western regions of Canada.20 On the other hand, more recently, chalkbrood was first identified in Queensland (Australia) in 1993, but in just a few years, it had spread to all Australian states.24 With this, the migratory nature of commercial beekeeping in North America and Australia is probably the most important factor contributing to the rapid spread of chalkbrood disease within these two continents,25 which is strengthening the role of predisposing factors for pathogen transference among colonies.
There is not a lot of information available on Asian honeybee chalkbrood. The pathogen has been found in Ascosphera cerana colonies in South Korea26 and is occasionally found in the same host species in China, mainly in drone brood. More specifically, the disease in China was first reported in 1990-1991, from the Zhejiang area.27 Later on, molecular diagnostic techniques confirmed the presence of this pathogenic fungus in China. This has further verified that Ascosphera cerana species of honeybees have been infected by this fungus for a long time. Regarding its trend, the disease has extensively spread to bee colonies in most parts of the country. Furthermore, the disease has recently become widespread throughout Asian beekeeping areas and is reported to be an economical disease of Ascosphera cerana honeybee colonies on the continent. However, there are no records of this fungal infection in any other Asian honeybee species other than Ascosphera cerana, and its impact on this species remains unknown.12
The presence of chalkbrood disease in Africa has been first reported from Tunisia and then from South Africa (Healthy, 1985).19 Furthermore, the presence of chalkbrood in Ethiopia was also reported in 2000-2001.28,29 These days, it has been confirmed that chalkbrood disease has been distributed in most parts of the beekeeping areas of the country and also in the whole African beekeeping region. As an indication of its broadening hosts besides Apis spp., Ascosphaera apis has also been isolated from the solitary bees.30
In general, the disease has recently been found in honeybee colonies around the globe, with a higher rate of distribution. This is due to the increased rate of food demand related to human activities and the higher use of different agrochemicals, which have weakened the immunity of the honeybees, together with the high rate of pollination targeting transportation and the longstanding viable spores of the fungus.15,31 Hence, appropriate pollination and other related human activities should have to be designed for minimal contribution and then controlled for distribution and transference.
Etiology of Chalkbrood Disease
A cross-sectional study design was conducted on lactating dairy cattle from November 2016 to April 2017 in Harar town. A cross-sectional survey and questionnaire were implemented to gather all relevant data for the dairy farms based on the information obtained from the Agricultural Bureau of Harari regional state about the dairy farms.
Morphology and reproduction of Ascosphaera apis: Ascosphaera apis belong to the heterothallic Ascomycota; here, sexual reproduction typically occurs between morphologically identical haploid partners, distinguished only by their mating type locus.32 This fungus, causing chalkbrood in honey bees, has a narrow host range and a unique infection route; it relies solely on sexual reproduction and has many host-specific adaptations.15 According to Spiltoir et al9 two Ascosphaera apis idiomorphs have been described as sexually dimorphic at the microscopic level, where hyphae of opposite mating types produce specialized reproductive structures. In nature, the development and growth of Ascosphaera apis strictly depend on the nutrients obtained from the honeybee brood. Even though the temperature and pH in the larval gut have a major effect on the viability and germination of fungal spores, germination requires very specific conditions that are found in the larval gut environment.33
On solid culture media with aerial, surface, and subsurface hyphae, Ascosphaera apis develops as a thick, white mycelium. The hyphae are septate, showing pronounced dichotomous branching. However, although the size of ascospores seemed to be a reliable and stable characteristic, many of the morphological characters used to identify Ascosphaera species vary greatly within each species. A typical feature of the genus Ascosphaera is the production of spherical spore cysts abundant in ascospores, forming spore balls.25 Both the shape and size of the ascospores as well as the size of the spore cysts differ between species. A mature ascoma measures in the range of 47-140 µm in diameter34 and contains numerous spore balls (asci). Spore balls enclosed within the cyst range from 7-19 µm in diameter and contain no visible outer membrane. The sporocyst contains smaller round bodies called spore balls (average 12 µm in diameter, Figure 1), and it is the ascospores (average 2.9×1.4 µm, Figure 1) found in the spore balls that the fungus is reaching at its most infective stage. Besides, scanning electron microscopy (SEM) revealed the presence of mitochondria and numerous ribosomes in the cytoplasm of the Ascosphaera apis mycelium.35
Figure 1. A. apis Ascogonial Development. Slides were Made from a Mixed Culture of Two Opposite Mating type Idiomorphs (ARSEF 7405 and 7406) Plated on a YGPSA Culture Medium and Incubated at 35 °C under 6% CO2
(A) Ascogonial primordia (arrow). (B) When in contact with the opposing mating type hypha, or shortly after, the nutriocyte starts to expand. (C) Development of a crozier (arrow). (D) Developing ascoma stained blue with Lectophenol Cotton blue dye. (E) Due to their resistance to Lectophenol Cotton Blue dye absorption, mature ascoma appear dark brown. The transparency of the ascoma walls allows observation of a number of small(10 μm) spherical-shaped asci. (F) Asci-containing fungus ascospores are expelled (arrow) when an ascoma ruptures, as seen in the figure. Scale bars (A-F) 10 μm.
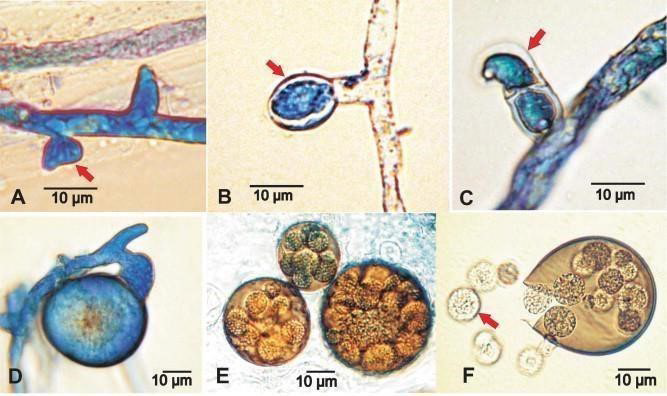
According to Heath19, neither coloration nor colony size or growth rate separate the two Ascosphaera apis mating types’ cultures from one another. On the other hand,9 described the two Ascosphaera apis idiomorphs as sexually dimorphic at the microscopic level, where hyphae of opposite mating types produce specialized reproductive structures (Figure 1 (A-E)).
Scanning electron microscopy also revealed that the wall of an ascoma is double-layered with a smooth outer surface.35 Moreover, scanning electron microscopy observations of Ascosphaera apis specimens revealed that mature ascospores are tightly packed inside the ascus. They have a thick spore wall and sporoplasm containing numerous ribosomes and mitochondria. The size of the individual ascospore is in the range of 2.7-4.0 µm and 1.4- 2.0 µm.34
Epidemiology of Chalkbrood Disease
Chalkbrood disease is typically most prevalent during the spring, given that fungal growth is enhanced in cool and humid (poorly ventilated) beehives.37,38 Concerning fungal strains,3 it was reported that various strains of Ascosphaera apis showed up to a 20-fold difference in the level of virulence. A high concentration of fungal spores in the colony substantially increases the chances of infection39 so the rate of disease incidence is likely dependent on a particular fungal strain’s level of ascospore production, the rate of spore germination, and the efficiency of spore dispersal.
Honeybee resistance against chalkbrood disease: The incidence of Ascosphaera apis significantly occurs in colonies that temporarily have insufficient numbers of adult bees to incubate their brood adequately around 35,36,37 °C.40 The development and course of the disease in bee colonies vary as they are affected by many factors such as infectiousness, individual immunity of the colony, genetic potential of the queen, environmental conditions, etc.25,41 As a result, Ascosphera mellifera ligustica brood was found to be less susceptible to Ascosphaera apis infection than Ascosphera mellifera mellifera and Ascosphera mellifera carnica brood.15 In addition, breeding for more disease-resistant bees provides an effective way to reduce the chemical treatment of diseases and reduce the need for pest control.7
Insects become infected by pathogenic fungi when their cuticles and peritrophic membranes are breached. However, the chemical components of the cuticle, such as waxes and unsaturated fatty acids, have potent antifungal activity.5 In addition, the biochemical environment of the midgut provides some defense against fungal food-borne pathogens. The by-product of melanin production is the release of reactive oxygen species that have cytotoxic antimicrobial properties.42
When the outer physical barriers are breached, the invasive fungi encounter a variety of physiological immune defenses through the activation of cellular and humoral immune reactions.3 Cellular immune responses begin immediately after an invasion is detected in the hemolymph, while antimicrobial peptides (AMPs) typically appear in the hemolymph some hours after infection.43 Phagocytosis and encapsulation are the most common defense mechanisms in bees against entomopathogenic fungi.44 Haemocytes can directly kill fungal spores and destroy other small foreign molecules by the phagocytic mechanism.45
Cellular immune responses begin immediately after an invasion is detected in the hemolymph, while AMPs typically appear in the hemolymph some hours after infection.44 However, genome analysis revealed that immune-related molecules are substantially less abundant in the bee genome than in its models, which could make them weaker for immediate and appropriate immune responses against infections. The chemical components of the cuticle in honeybee brood, on the other hand, such as waxes and unsaturated fatty acids, are also noted to have potent antifungal activity.5
Furthermore, some degree of protection is provided by the social organization of the honeybee colony. Honeybees have developed several types of behavior in order to avoid, control, or eliminate an intruding pathogen.46 To clarify this point, the hygienic behavior of honeybee colonies, which were subsequently provided with pollen cakes containing Ascosphaera apis, has been evaluated, and we have come to the conclusion that most hygienic colonies have been able to control the disease better.39
Pathogenesis of Chalkbrood Disease
Ascosphaera apis spores, or ascospores, are historically thought to be the main cause of brood infection. Early research revealed that two ways of becoming infected by ascospores could occur: through the cuticle and by ingestion. It is now commonly acknowledged that A. apis spores cannot germinate on the cuticle of larvae; hence, larvae must swallow them in order to contract the infection.40 Ascosphaera apis can infect broods of any caste (workers, drones, or queens). According to Harbo41 larvae are most susceptible at 3 to 4-days of age, while others report that 1 to 2-day-old larvae are highly susceptible as well. While adult bees are not susceptible to this pathogen, they can transmit the disease within and between beehives. Transmission of infectious materials between adult bees within the colony appears to be via food sharing.47 Fungal spores can be carried by foraging bees and passed onto larvae by nurse bees feeding them contaminated food. Transmission between managed colonies is mostly beekeeper-assisted due to contaminated materials. Any hive material contaminated with fungal spores will act as a longlasting source of infection since spores can build on all components of the beehive and in all beehive products (such as foundation wax, stored pollen, and honey) and remain viable for at least 15-years.39
Spores consumed by the honey bee larvae germinate in the lumen of the gut, probably activated by CO2 .41 Infected larvae rapidly reduce food consumption and then stop eating altogether. Recently identified are several enzymes produced by Ascosphaera apis, some of which are implicated in assisting the pathogen in penetration of the peritrophic membrane of the bee larval midgut.48 After penetrating the gut wall, the fungal mycelium grows inside the body cavity, eventually breaking out through the posterior end of the larva49 (Figure 2(A-C)). Death occurs as a result of mechanical and enzymatic damage, disruption of hemolymph circulation, and general toxicoses.3 Ascosphaera apis vegetative growth extends from the posterior end to the anterior end of the larva, eventually covering the entire larva with a thick layer of white mycelium (Figure 2D). Later, fungal growth is mottled with brown or black spots due to the production of ascomata that may vary in size and color.
Figure 2. In vivo Bioassay
A) Control bee larva. (B) Bee larvae 72 h post-infection. Arrows denote whitecolored mass formed by fungal mycelium growing under the skin. (C) Bee larvae 78 h post- infection showing clinical signs of chalkbrood (arrows). (D) Bee larvae 6-days post-infection.
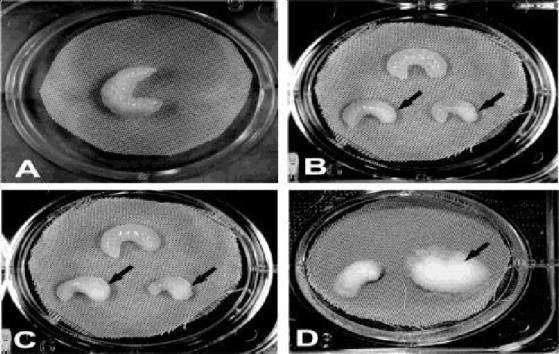
Honey bee cadavers are usually found stretched out in the cells in an upright condition, swollen to the size of the cell.50 Eventually, cadavers dry and form so-called chalkbrood mummies (Figure 3), which may be white or black, depending on whether or not ascospores are present. Each black mummy contains about 108-109 ascospores.25 Microscopic examination of white mummies revealed primarily cellular debris and mycelial fragments, but no detectable ascomata or ascospores.
Figure 3. Chalkbrood Mummies
Chalkbrood mummies are white, brown, or black

It has been proposed that mycelia of a single mating type are the cause of white mummies. This seems questionable given that it has never been shown that mycelia are infective. Nor has it been shown51 that a single mating type is capable of producing asexual spores. In addition, both mating-type idiomorphs were routinely isolated from white mummies, and fungal cultures originating from single white mummies and grown separately consistently produced ascospores in culture.20 Others hypothesized that one of the Ascosphaera apis mating types may inhibit the growth of the other or that they may not be equally distributed in the environment. It may be true that there is an inhibition of one mating type by the other, but that does not seem to occur when opposite mating types are grown together in the laboratory, although their behavior may be different in the natural environment. What seems more likely is that younger mummies are white, and given enough time and the proper conditions, they will become black due to the eventual development of ascospores.25
Sign of Chalkbrood Disease
The following signs characterize clinical evidence of the disease: Infection is more commonly found in worker and drone larvae than in queen larvae.52 When uncapped, dead larvae at first are somewhat fluffy white, swollen, and sponge-like and may take on the hexagonal shape of the cell. The “mummies” are described as becoming firm and chalk-like later on. They may remain white or, if the fungus produces fruiting bodies, turn gray or black. The mummies stay white if only one strain of the fungus is present, but when both strains are present, the formation of fruiting bodies causes the mummies to turn gray or black. By this stage, the capping hive has frequently been removed by the bees.40
Diagnosis of Chalkbrood Disease
Diagnosis of the disease in a honey bee colony is generally made on the basis of white, black, or gray mummies at the hive entrance, on the bottom board, or in sealed and unsealed brood cells.47,53 Clinical evidence of the disease is characterized by dead larvae in capped cells, infection predominantly found in worker and drone larvae (rather than in queen larvae), small perforations in otherwise normal cell capping, initially fluffy white, swollen, and sponge-like larvae in uncapped cells, and later hard and chalk-like “mummies” (either whitish or grey/black). The mummies remain whitish if they are infected with only one strain of the fungus, but will turn grey or black when infected with both strains of the fungus as a result of the production of fruiting bodies.25
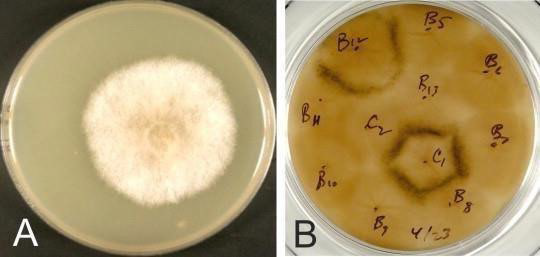
The microscopic presence of spore cysts is usually sufficient to make a diagnosis. These spore cysts, which are about 60 µm in diameter, contain smaller round bodies known as spore balls (average 12 µm in diameter). The most infective stage of the fungus, the spores (2.9×1.4 µm), are found in these spore balls.52 In samples where only white “mummies” have been submitted and spore-producing bodies cannot be detected microscopically, it may be necessary to grow the fungus in vitro. 24 For identification purposes, a heat treatment of the inoculum is routinely performed prior to plating to kill non-spore-forming microbes that are routinely found in chalk brood mummies.54 Fungal growth is typically visible on plates in 2 to 3-days (Figure 4A). After 4 to 6-days of incubation, black specks of ascomata will appear on the mycelial lawn. When strains of different mating types are inoculated onto plates a slight distance apart, ascomata will appear as characteristic black lines where the mycelia intersect (Figure 4B).
Figure 4. A. apisculture
(A) Mixed Ascosphera apisidiomorphs cultured on solid YGPSA culture medium at 35 °C under 6% CO2 . (B) Mating type test. Characteristic black lines of ascomata formed at the interface of the opposite mating type colonies can be observed when fungal colonies are plated at a distance.25
Control and Management of Chalk Brood Disease
Over the years, a number of alternative strategies have been developed and implemented to control chalkbrood disease.25 Some of those are described below and are most effective and widely accepted by beekeepers. These methods include chalkbrood-resistant bee lines, improved management and sanitation practices, and the use of ecologically safe natural products.55,56
Improving genetic stock: Hygienic behavior is considered the primary mechanism of honey bee resistance to a variety of brood diseases.57,58 Therefore, replacing a queen with good hygienic stock has become one of the most common practices for dealing with chalkbrood. There appears to be a strong genetic component to chalkbrood resistance in hygienic bees.47 Over the past several decades, much research has been focused on enhancing the hygienic behavior of bees through breeding.59,60 Colonies exhibiting significant hygienic behavior have reduced the numbers of fungal spores in stored food and comb wax.4 Recent studies showed that the genetic basis of hygienic behavior involves a number of genes whose products interact in a complex way and demonstrated that increased genetic diversity in bees may play a significant role in lowering the incidence of disease outbreaks.61,62
Management and sanitation: Management and sanitation strategies are directed toward helping bees fend off infection or avoid infection in the first place. These practices include extra feeding to enhance the nutritional and health condition of bees, maintaining clean, well-ventilated hives, utilizing clean equipment, changing store and brood combs annually, and avoiding comb transfers between colonies.39 An additional benefit of replacing old combs is that they often contain residual pesticides.55,63
Irradiation was also tested to sterilize wax and honey. At the optimum level of radiation (10 k Gray), there were no negative effects detected on wax composition, though some physicochemical effects were observed in honey, including decreases in enzymatic activity, changes in color, and leakage from frames.64 However, the accessibility of radiation facilities is the limiting factor in utilizing this technology.65 demonstrated that Ascosphaera apis spores can be killed by incubation of honey for 8-hours at 65 °C or at 70 °C for 2-hours in water baths. The heat also increases the level of the harmful chemical hydroxymethylfurfural (HMF) and considerably reduces beneficial enzyme activity (e.g., diastase) in honey.66
Control of chalkbrood disease with natural products and microorganisms: A welcome replacement for synthetic fungicides for the management of the chalkbrood fungus would be natural chemicals. In an effort to control chalkbrood, a wide variety of chemicals have been tested on A. apis in culture and in honey bee colonies. Some of them include natural plant-derived antimicrobial products.67,68 The antifungal activity of many natural compounds has been tested. Essential oils containing citral, geraniol, and citronellal were reported to have the best inhibiting effect on fungal growth in vitro.51 These findings need to be tested in field studies to evaluate the efficacy of the most active products in beehives. A broad-spectrum antimicrobial compound (lysozyme) was tested in field studies in Canada and showed promise for the control of chalkbrood in bee colonies.17 Numerous microbes associated with honey bees, such as certain Penicillum, Aspergillus, and Bacillus species, showed inhibiting effects on the growth of Ascosphaera apis in culture.4,11
Chalkbrood Disease in Ethiopia
The survey on chalkbrood illnesses in Ethiopia began in 2000, and in 2001, reports indicated the diseases’ presence in the Holeta study regions.70 There is no comparison of the prevalence of chalkbrood illnesses among bee colonies in the same apiary or among bee colonies in other apiaries. However, two private beekeeper-owned apiaries near the Holeta bee research center reported the highest infestation rate. The study found that different apiary sites had varying levels of disease infestation, with prevalence rates ranging from 0 to 100% and an overall infestation level of 17.4%. Study in Addis Abeba apiary locations. In addition, 43% of the bee colony was reported by Desalegn et al71 to have illnesses. Additionally, 28 reported the presence of the illnesses.
In Ethiopia, the geographical distribution of chalkbrood diseases in honey bees was recorded. The study reported an infection rate of 37.12%, 19.89% and 17.93% and a distribution rate of 87.50%, 56.56% and 33.33% in Amhara, Oromia, and BenshangulGumuz, respectively. Also, the infestation and distribution rate of the chalkbrood diseases are unequal72 reported distribution rates (100%) in West Gojjam and 95.42% in Jimma out of 33 Woredas sampled for the study of the diseases.
CONCLUSION AND RECOMMENDATIONS
Over the past several decades, much research has been done to control and improve honeybee lines through selection as a mechanism against chalkbrood disease and other entomopathogenic infections. However, a better understanding of chalkbrood and other pathogenic diseases’ epidemiology, which will lead to improved management tactics against potentially prevalent diseases, has not yet been obtained and popularized.
Further work needs to be carried out to identify and test candidate chemicals for their ability to control chalkbrood and whether they leave residues in honeybee products in field conditions. The propagation of chalk-brood-resistant bees in the beekeeping industry needs to be further investigated. Beekeepers should also be trained to assess whether their colonies have chalkbrood resistance traits. These chalk-brood-resistant bees could then be propagated, provided they also have the necessary production capabilities.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









