INTRODUCTION
Infectious bursal disease (IBD), also known as Gumboro disease, has been a great concern for the poultry industry worldwide. It was first reported in broiler flocks in the area of Gumboro, Delaware in 1957.1
Infectious bursal disease is caused by the infectious bursal disease virus (IBDV), which is an acute and very contagious disease that affects growing chickens between the ages of 3 to 6 weeks.2 It is caused by a virus that is a member of the genus Avibirnavirus of the family Birnaviridae3 which is characterised by the destruction of lymphocytes in the bursa of fabricius.4 It is a non-enveloped, double-stranded ribonucleic acid (RNA) and bisegmented virus, that is, segment A and B.5 There are two serotypes of IBDV, namely serotypes 1 and 2. In chickens, serotype 1 is pathogenic and consists of three viral strains: classical (ca), very virulent (vv), and variant (va) IBDV. In chickens, serotype 2 is nonpathogenic). There are two serotypes of IBDV, namely serotypes 1 and 2. In chickens, serotype 1 is pathogenic and consists of three viral strains: classical (ca), very virulent (vv), and variant (va) IBDV. In chickens, serotype 2 is not pathogenic.1
Infectious bursal disease is a commonly encountered lymphocytolytic disease that adversely affects the defence mechanism of birds and results in immunosuppression and a failure to develop satisfactory immunity.6
Infectious bursal disease virus infections, clinical signs, organ lesions, and immuno-suppression correlate with the status of immunity, age, and genetic background of affected chickens and with the virulence of the infecting virus strain. After an incubation period of 2-3-days, young chickens show symptoms of ruffled feathers, watery diarrhea, trembling, severe prostration, severe depression, vent picking, the presence of urate stains on the vent, dehydration, loss of appetite, and elevated water consumption, and death may follow 1-3-days later. Mortality will peak and recede, usually in a period of 5-7-days.7
In most cases, a preliminary diagnosis can be made based on flock history, clinical signs, and post-mortem (necropsy) examinations. A necropsy will typically reveal changes in the Fabricius bursa, such as swelling, oedema, haemorrhage, the presence of a jelly serosa transudate, and eventually bursal atrophy.8
Various diagnostic methods, such as the virus neutralisation test (VNT), enzyme-linked immunosorbent assay (ELISA), and agar gel immunodiffusion test (AGIDT), are used infrequently to detect IBDV, whereas molecular techniques, such as reverse transcriptase-polymerase chain reaction (RT-PCR), are frequently used to detect viruses from field samples.9 Laboratory confirmation was achieved by virus isolation followed by its serological assay and histopathological examination of the affected bursa.10 Isolation of the viruses is laborious, nonspecific, and time-consuming. The more frequently used molecular method is the reverse transcription polymerase chain reaction (RT-PCR).11
The main effective way to control IBD is vaccination, and different vaccination programmes are regularly implemented globally, including in Africa.12 Vaccination has become the principal control measure of IBDV infection in chickens since the virus is resistant to different physical and chemical methods of decontamination.13 Vaccines and vaccination programmes vary widely depending on several local factors (e.g. type of production, level of biosecurity, the local pattern of disease, the status of maternally derived antibodies, vaccines available, costs and potential losses).6
The objectives of this paper are:
• To highlight various commonly used diagnostic methods for infectious bursal disease.
• To review advances made in diagnostic methods and vaccination strategies for IBDV, with special emphasis on the strengths and weaknesses of each of those techniques.
INFECTIOUS BURSAL DISEASE
Infectious bursal disease is an acute and highly contagious viral infection of immature chickens. IBD is characterised by the destruction of lymphocytes in the bursa of Fabricius and, to a lesser extent, in other lymphoid organs. Infectious bursal disease virus is the cause of infectious bursal disease, also known as “Gumborodisease”.14,15
Etiology
Infectious bursal disease virus is a double strand RNA virus (dsRNA) and a non-enveloped, icosahedral capsid with a bi-segmented genome.16 The larger segment, A, is 3261 nucleotides long and contains two open reading frames (ORFs) that encode four viral proteins known as VP2, VP3, VP4, and VP5. The smaller segment B encodes only VP1, which has polymerase activity. The two viral proteins, VP2 and VP3, are structural proteins that make up the viral capsid. The epitopes responsible for the induction of neutralising and protective antibodies are found in the VP2 protein (Figure 1).17
Figure 1. Structure of Infectious Bursal Disease Virus Particles
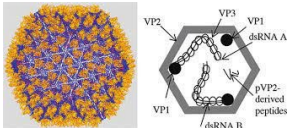
Mutations in the IBDV genome have impacted antibody recognition and led to variations in the antigenicity, immunogenicity, virulence, and tropism of circulating infectious bursal disease virus strains.18
General Characteristics of the Infectious Bursal Disease Virus
Two serotypes of infectious bursal disease, serotypes one and two, have been recognised as having considerable antigenic variation within each serotype.19 It is a naked virus, devoid of an envelope, known for its resistance to physical and chemical agents and to potential of hydrogen (pH) conditions of 2-11, but it is inactivated at pH 12. Due to this ability of stability and hardiness, it persists in poultry premises even after thorough cleaning and disinfection for up to 4-weeks in the bone marrow of infected chickens. The virus has been shown to remain infectious for 122-days in a chicken house and for 52 days in feed, water, and faeces.20
Pathogenesis of Infectious Bursal Disease
Following host entry via oral ingestion or inhalation, IBDV may bind to host cell proteins such as N-glycosylated polypeptide (s) expressed on the cell membrane of immature IgM+ B-cells during the viral entry process. It is transported by infected macrophages to the bursa of Fabriciaus, where the virus undergoes intracytoplasmic replication in IgM+ B lymphocytes.21 Due to its short incubation periods, which range from 2 to 3-days, a pore-forming peptide of the virus (pep46), which is associated with the outer capsid of the IBDV particle, may facilitate viral entry into the cytoplasm of infected cells.22
Mature and competent lymphocytes will expand as a result of stimulation by the virus, whereas immature lymphocytes will be destroyed. The bursa is infiltrated by heterophils and undergoes hyperplasia of the reticulo-endothelial cells and of the interfollicular tissue.23
Diagnosis
Diagnostics of infectious bursal disease involves consideration of the flocks’ history and of the clinical signs and lesions. Clinical manifestations and post-mortem findings of affected birds may aid in the diagnosis of IBD disease, but laboratory diagnosis is necessary for its confirmation.24 Chickens less than 3-weeks of age present no clinical signs of disease, but chickens greater than 3-weeks of age present clinical signs.25
Gross and histopathological examinations of the bursa are used to diagnose IBD in young chickens or in those having maternal antibodies.20 However, other methods used in diagnosis include isolation and detection of IBDV using embryonated chicken eggs, cell culture, RT-PCR and serology, such as virus neutralisation, indirect ELISA, and agar gel immune diffusion test.26
Virus isolation:
IBDV can be isolated (grown) in chicken embryos and primary cell cultures, especially chicken fibroblast cells. Isolation and identification of the agent provide the most certain diagnosis of IBD, but they are not usually attempted for routine diagnostic purposes as the virus may prove difficult to isolate.10
(a) Isolation of virus in embryos:
The most sensitive diagnostic method for virus isolation is the inoculation of bursal homogenates from IBDV-infected chickens into the chorioallantoic membrane of 9-10-days old embryonated specific-pathogen-free (SPF) chicken eggs. The most sensitive route of inoculation is the chorio allantoic membrane; the yolk sac route is also practicable.27 It is especially important for wild-type IBDV, which usually does not replicate in conventional cell culture, can also be regenerated by the reverse genetics approach, but can grow in embryonated chicken eggs.26 Some strains grow well in embryos but are not readily adapted to grow in chicken embryo fibroblasts or chicken embryo kidney. Variant viruses, however, do not kill the embryos but cause embryo stunting, discoloration, splenomegaly, and hepatic necrosis.20
(b) Isolation of virus in cell culture:
In about 3-5-days, IBDV grows in chicken embryo fibroblasts and produces cytopathogenic effect (CPE) characterised by the appearance of round retractile cells.21 Because the virus is difficult to culture, IBDV isolation in cell culture is not commonly used as a diagnostic test. In cell cultures, some field strains did not grow at all.28 In tissue culture, wild-type infectious bursal disease virus strains, particularly the most virulent strain, do not grow. A comparison of the genome sequences of wild-type and tissue culture adapted IBDV strains revealed several mutations that may be responsible for IBDV invitro growth in tissue culture.29
Serological characterization:
Typically, blood can be collected from the wing vein, allowed to clot, and serum separated by centrifugation and stored at -20 °C until tested. Agar gel immuno diffusion (AGID) test, ELISA, and viral neutralisation (VN) test are the most commonly used serological tests for detecting IBDV.30
(a) Agar gel immunodiffusion test:
The AGID test is the most effective serological test for detecting specific antibodies in serum or viral antigens in bursal tissue.23 The test is specific because it cannot produce false positives but can produce false negatives. AGID can detect the presence of IBDV antigen in bursal tissue for 5-6- days after infection.31
Blood samples should be taken early in the course of the disease, and repeat samples should be taken 3-weeks later. Because the virus spreads rapidly, only a small proportion of the flock needs to be sampled. Usually, 20 blood samples are enough. For detection of antigen in the bursa of fabricius, the bursae should be removed aseptically from about ten chickens at the acute stage of infection. The bursae are minced using two scalpels in a scissor movement, then small pieces are placed in the wells of the AGID plate against known positive serum. Freeze–thaw cycles of the minced tissue may improve the release of IBDV antigens from the infected bursal tissue.3
(b) Virus neutralization tests:
Viral neutralisation tests are carried out in cell culture. The test is more time-consuming and costly than the AGID test, but it is more sensitive for detecting antibody.23 This sensitivity is not required for routine diagnostic purposes, but may be useful for evaluating vaccine responses or differentiating between IBDV 1 and 2 serotypes. To reduce test-to-test and operator-to-operator variation, a standard reference antiserum may be included with each batch of tests, and the titer of the virus suspension must be reassessed in each new experiment using a sufficient number of repeats (wells) per virus dilution.3
(c) Enzyme-linked immunosorbent assay:
The ELISA is the most commonly used test for the detection and quantification of IBDV antibodies to check for response to vaccination, natural field exposure, and decay of maternal antibody titer. It is economical, simple, and quick to test a large number of samples at the same time and is adaptive to automation by computer software.20
The test sera are diluted according to the established protocol or kit instructions, and each is dispensed into the requisite number of wells. After incubation under the appropriate conditions, the serum is discarded from the plates, and the wells are washed thoroughly. Wells are dispensed with anti-chicken immunoglobulins conjugated to an enzyme and the plates are again incubated as appropriate. The plates are emptied and rewashed before a substrate containing a chromogen that gives a colour change in the presence of the enzyme used is added to the plate. After a final incubation step, the substrate/chromogen reaction is stopped by the addition of a suitable stopping solution, and the colour reactions are quantified by measuring the optical density of each well. The sample to positive (S/P) ratio for each test sample is calculated.3
Identification by molecular method:
Molecular detection and characterisation, involving sequencing and phenotypic and genotypic analyses, have been utilised in the diagnosis of IBD. This method can detect the genome of IBDV, which is unable to grow in cell culture or embryonated eggs because it is unnecessary to grow the virus before amplification, even when the virus is present in a very minute quantity and has lost its infectivity.32 The classical methods for molecular characterization and differentiation of IBDV field isolates include RT-PCR, restriction fragment length polymorphism (RFLP), nucleotide sequence analysis, and quantitative real-time RT-PCR (qRT-PCR).3
Reverse transcriptase polymerase chain reaction offers a rapid, highly sensitive and specific test for the confirmative diagnosis of the disease which would help in controlling the disease, thereby reducing the economic losses significantly. RT-PCR in combination with restriction enzyme analysis allows the rapid identification of IBDV. Nucleotide sequencing of RT-PCR products is widely used for further characterization of IBDV strains.18
The VP2 gene of IBDV contains a variable region, which suggests the potential of this region for differentiation of IBDV strains. RT-PCR followed by digestion with multiple restriction enzymes or RFLP and nucleotide sequencing of the VP2 gene have been used for the differentiation of IBDV strains. The molecular differentiation of IBDV strains using VP2 has been improved by the use of labelled probes in real-time RT-PCR.33
Post-mortem findings:
Pathological change observed at the bursa of Fabricius is characteristic and histopathological investigations combined with the demonstration of viral antigen by immunohistochemistry confirm an IBDV infection.34 Diagnostic lesions include muscle haemorrhages and bursal enlargement. Pathognomonic gross lesions are observed in the bursa of Fabricius, which show doubling in size with a yellowish gelatinous film that may surround it and sometimes haemorrhages may be seen on the surface of it (Figure 2).1
Figure 2. Gross Lesions Observed in the IBDv Affected Bursa of Broiler Chicken

Histopathology examination:
The lymphoid structures primarily affected by IBDV are: BF, spleen, thymus, Harderian glands, caecal tonsils, gut-associated lymphoid tissue (GALT) and head-associated lymphoid tissues (HALT). Lymphocytic degeneration and necrosis in the medullary region of the BF at 1-day post-infection are the first signs.6
Microscopic examination of tissues shows moderate haemorrhages in the muscles and kidneys, and the spleen shows moderate lymphoid depletion in the lymphoid nodules. There is marked interfollicular oedema and depletion of 13 lymphocytes from the lymphoid nodules in the BFs. Other lymphoid nodules of the BF show degeneration and necrosis of lymphocytes and cystic cavitations with heterophil infiltrates.5
Vaccine and Vaccination against IBD
Vaccination of chickens with high quality vaccines is the primary method of control of many poultry infectious diseases, including IBD (Gumboro) disease.35
With a proper vaccination schedule, it is possible to protect chickens. Many studies have identified rational vaccination schedules and strict biosecurity measures as critical tools for IBD management.36 Despite the fact that different types of IBD vaccines are being developed, two of them are commonly used for IBD control. These are either live attenuated or inactivated oilemulsion adjuvanted vaccines.8 Currently, plant-based vaccines are available, and a live recombinant vaccine expressing IBDV antigens has also been approved.17
Live-attenuated vaccines:
Live viral vaccines can activate the target host’s immune system. They have the ability to replicate and induce both cellular and humoral immunity. They do not require an adjuvant to be effective and can be given to chickens in large quantities, but they may have unfavourable side effects. Horizontal and vertical transmission (though not in the case of IBD vaccines), reversion to virulence, and vaccine reactions that may result in disease or production loss are examples of these. In general, the live IBDV vaccines used in the poultry industry have been attenuated through serial passage in tissue culture, eggs, or embryo-derived tissues, with the goal of maintaining the immune response induced by the parent virus while attenuating the vaccine virus’s ability to cause disease.37
Inactivated vaccines:
Inactivated IBD vaccines are mostly formulated as water-in-oil emulsions, usually combining several antigens, and have to be injected into each bird. It has been observed that inactivated IBD vaccines are able to induce IBDV-specific T-cell and inflammatory responses in chickens. It has been reported that inactivated IBD vaccines must have either a high or an optimised antigenic content in order to induce in breeders an immunity that helps protect the progeny from infection by variant IBDV strains.15
Killed-virus vaccines with an oil adjuvant are frequently used to increase maternal antibody levels and confer longer-lasting immunity in breeder hens. The concentration and antigenic specificity of the vaccine strain may influence the duration and uniformity of this immunity. Because these vaccines are not ideal for inducing a primary antibody response, they are most effective in chicks that have been “primed” with a live virus vaccine or have been naturally infected through field exposure to IBDV.5 Many oil adjuvant vaccines now include both classic and variant IBDV strains. Killed-virus vaccines are administered by subcutaneous or intramuscular injection between the ages of sixteen and twentyweeks.12
New generation or genetically-engineered IBD vaccines:
Genetically engineered IBD vaccines have also been developed as a result of improved understanding of the molecular structure and immunology of IBDV. The viral capsid protein VP2, encoded by genomic segment A and derived from a large precursor protein VP0 by a series of proteolytic processes, carries immune determinants that control antibody-dependent neutralisation and protection. In general, these could be divided into two categories based on their replicative nature upon delivery into the chicken.38
(a) Non-replicative IBD vaccines:
Immunisation with deoxyribonucleic acid (DNA) or subunit vaccines involves the use of nonreplicating IBDV for the induction of an immune response in birds. DNA vaccination is based on direct inoculation of plasmid DNA encoding a target immunogen gene into subjects of study.39 Under the influence of a mammalian promoter, the target genes were expressed to produce proteins in vivo that are able to induce immune responses in the injected host. Repeated injections of DNA vaccines carrying the IBDV genes, either the polyprotein genes or the gene of VP2 alone, were shown to protect the chickens from challenge virus.40
However, the presence of maternally derived antibody (MDA) could affect the efficacy of DNA vaccines, and a high dose of DNA vaccines was required to overcome the interference of MDA and induce an immune response in chickens. It was shown that a booster vaccination with inactivated IBD vaccine after priming with DNA vaccine provided better and higher protection to the chickens compared to injection with DNA vaccines alone.41
(b) Replication-competent IBD vaccines:
Replication-competent viral vectors have been utilised to express and deliver immunogens of interest to chickens. In contrast to DNA and subunit vaccines, vaccination by live recombinant virus vectors employs the use of live and replicating viruses to produce IBDV antigen upon in vivo infection. They have been shown to elicit both humoral and cellmediated immune responses in chickens. As they could persistently infect the chickens, the potential for having a long-term protective immunity is high.42
Besides, the recombinant viral vectors are less sensitive to MDA and could therefore evade neutralisation by the maternal anti-IBDV antibody.12 Several viruses have been engineered to express the VP2 protein of IBDV. This includes fowlpox virus, fowl adenovirus, Marek’s disease virus, Newcastle disease virus, and avian adeno-associated virus, among others.42 The VP2 protein expressed in vivo from these various studies has been shown to confer from partial to full protection to vaccinated chickens from mortality, although they do not prevent the damage to the bursa.43
Plant-produced IBD vaccines:
The plant-based expression system is becoming a more popular alternative platform for animal vaccine production and development.44 Because VP2 capsid protein is one of the most important pathogenic agents in poultry, a plantbased expression system using the stable,45 transient,46 or chimeric viral particles47 approach was used to create an IBD vaccine containing it. Transgenic rice expressing the VP2 protein was shown to protect the chickens from challenge following oral immunisation.16
Recently, the VP2 protein of IBDV has been transiently expressed in Nicotiana benthamiana leaves and extracted for subunit vaccination in chicken.46 The recombinant VP2 protein emulsified in oil adjuvant, injected intramuscularly to chicks at 18-days of age and followed by booster doses after 22 and 35-days, was shown to induce the production of anti-IBDV antibodies with neutralising ability.47
In ovo vaccination and post-hatch vaccination:
In ovo vaccination and post-hatching vaccination technology have recently been developed to deliver live vaccine into eggs during the incubation period. After 18-days of incubation, the complex of live vaccine viruses and IBD antibodies is injected in ovo. When the chicks are about 7-days old, the vaccine virus is released and the eggs hatch. The problem of maternally derived IBD antibodies is thus solved, and the chicks are effectively immunized.27 Compared to posthatch vaccination, in ovo injection of a live intermediate vaccine allowed faster recovery from bursa lesions, although both methods exhibited similar protection against challenge.48 Although in ovo vaccine delivery is an appealing alternative to post-hatch vaccination, several factors, including dosage, virulence, and efficacy, must be properly optimised before pursuing large-scale vaccinations.49
CONCLUSION AND RECOMMENDATIONS
Infectious bursal disease is caused by the IBD virus that affects the immune cells of chickens. It is mainly a disease of young chickens between 3-6-weeks old and causes secondary problems due to the effect of the virus on the bursa of Fabricius. Diagnosis of IBD depends on clinical signs, differential diagnosis, gross lesions, histopathological lesions, virus isolation, serological and molecular diagnosis. Isolation and identification of the agent can deliver the most confident diagnosis of infectious bursal disease.
From the recommended serological tests for IBD virus, AGID is the simplest but least sensitive, whereas ELISA is a rapid and sensitive method, but it cannot differentiate serotypes. The virus neutralisation test is the gold standard and the only serologic test that differentiates antibodies of two serotypes and is sensitive, but it is more laborious and expensive than the AGID. Molecular Identification Reverse Transcription-Polymerase Chain Reaction is used to detect IBDV without considering the viability of the virus by working on VP2 found in segment A of the viral capsid.
Vaccination is the principal control measure of IBDV infection in chickens. Of the available vaccines, the live vaccine is the most protective and widely used IBD vaccine. Vaccination strategies in ovo, at-hatch or on-farm vaccinations, determine the choice of vaccines used on the farm. Therefore, based on the above conclusion, the following recommendations are forwarded: The virus neutralisation test is the most sensitive, but it is laborious and time-consuming.
• The reverse transcriptase polymerase chain reaction has been the most simple and sensitive molecular diagnostic technique.
• Further cost-benefit analysis must be conducted on more safe and effective IBD vaccines that are affordable and readily available.
• Ivo vaccination will be the best vaccination strategy against infectious bursal disease.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.







