1. Patel V, Flisher A, Hetrick S, McGorry P. Mental health of young people: A global public-health challenge. Lancet. 2007; 369(9569): 1302-1313. doi: 10.1016/S0140-6736(07)60368-7
2. Cassidy SA, Bradley L, Bowen E, Wigham S, Rodgers J. Measurement properties of tools used to assess suicidality in autistic and general population adults: A systematic review. Clin Psychol Rev. 2018; 62: 56-70. doi: 10.1016/j.cpr.2018.05.002
3. Horowitz LM, Wang PS, Koocher GP, et al. Detecting suicide risk in a pediatric emergency department: Development of a brief screening tool. Pediatrics. 2001; 107(5): 1133-1137. doi: 10.1542/peds.107.5.1133
4. Cappelli M, Gray C, Zemek R, et al. The HEADS-ED: A rapid mental health screening tool for pediatric patients in the emergency department. Pediatrics. 2012; 130(2): e321-e327. doi: 10.1542/peds.2011-3798
5. Ballard ED, Cwik M, Van Eck K, et al. Identification of at-risk youth by suicide screening in a pediatric emergency department. Prev Sci. 2017; 18(2): 174-182. doi: 10.1007/s11121-016-0717-5
6. Zbozinek T, Rose R, Wolitzky-Taylor K. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety. 2012; 29(12): 1065-1071. doi: 10.1002/da.22026
7. Masten AS. Adolescent Psychopathology and the Developing Brain. Integrating Brain and Prevention Science. Romer D, Walker EF (Eds). New York, USA: Oxford University Press. 2007.
8. Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, Wagster MV. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 2010; 9(2): 138-139. doi: 10.1016/S1474-4422(09)70335-7
9. Sareen J, Cox B, Afifi T, et al. Anxiety disorders and risk for suicidal ideation and suicide attempts: A population-based longitudinal study of adults. Arch Gen Psychiatry. 2005; 62(11): 1249-1257. doi: 10.1001/archpsyc.62.11.1249
10. Singh H, Graber ML. Improving diagnosis in health care-the next imperative for patient safety. N Engl J Med. 2015; 373(26): 2493-2495. doi: 10.1056/NEJMp1512241
11. Lahti A, Harju A, Hakko H, Riala K, Räsänen P. Suicide in children and young adolescents: A 25-year database on suicides from Northern Finland. J Psychiatr Res. 2014; 58: 123-128. doi: 10.1016/j.jpsychires.2014.07.020
12. Kim H, Lim C, Kaang B. Neuronal mechanisms and circuits underlying repetitive behaviors in mouse models of autism spectrum disorder. Behav Brain Funct. 2016; 12(1): 3. doi: 10.1186/s12993-016-0087-y
13. Zike I, Xu T, Hong N, Veenstra-VanderWeele J. Rodent models of obsessive-compulsivedisorder: Evaluating validity to interpret emerging neurobiology. Neuroscience. 2017; 345: 256-273. doi: 10.1016/j.neuroscience.2016.09.012
14. Ricketts EJ, Snorrason Í, Kircanski K, et al. A latent profile analysis of age of onset in pathological skin picking. Compr Psychiatry. 2018; 87: 46-52. doi: 10.1016/j.comppsych.2018.08.011
15. Chamberlain SR, Odlaug BL, Boulougouris V, Fineberg NA, Grant JE. Trichotillomania: Neurobiology and treatment. Neurosci Biobehav Rev. 2009; 33(6): 831-842. doi: 10.1016/j.neubiorev.2009.02.002
16. Zetterqvist M. The DSM-5 diagnosis of nonsuicidal self-injury disorder: A review of the empirical literature. Child Adolesc Psychiatry Ment Health. 2015; 9: 31. doi: 10.1186%2Fs13034-015-0062-7
17. Flessner C, Knopik V, McGeary J. Hair pulling disorder (trichotillomania): Genes, neurobiology, and a model for understanding impulsivity and compulsivity. Psychiatry Res. 2012; 199(3): 151-158. doi: 10.1016/j.psychres.2012.03.039
18. Fox MD. Mapping symptoms to brain networks with the Human Connectome. N Engl J Med. 2018; 379: 2237-2245. doi: 10.1056/NEJMra1706158
19. Glasser MF, Coalson TS, Robinson EC, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016 Aug 11; 536(7615): 171-178. doi: 10.1038/nature18933
20. Gardner D, Akil H, Ascoli G, et al. The neuroscience information framework: A data and knowledge environment for neuroscience. Neuroinformatics. 2008; 6(3): 149-160. doi: 10.1007/s12021-008-9024-z
21. Lijie Wang, Jinping Xu, Chao Wang,Jiaojian Wang. Whole brain functional connectivity pattern homogeneity mapping. Front Hum Neurosci. 2018; 12: 164. doi: 10.3389/fnhum.2018.00164
22. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000; 23: 155-184. doi: 10.1146/annurev.neuro.23.1.155
23. Romer D, Reyna VF, Satterthwaite TD. Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Dev Cogn Neurosci. 2017; 27: 19-34. doi: 10.1016/j.dcn.2017.07.007
24. Consortium B, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018; 360(6395). doi: 10.1126/science.aap8757
25. Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997; 385: 533-538. doi: 10.1038/385533a0
26. Douglas RM, Goddard GV. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975; 86(2): 205-215. doi: 10.1016/0006-8993(75)90697-6
27. Guo-li Ming, Hongjun Song. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011; 70(4): 687-702. doi: 10.1016/j.neuron.2011.05.001
28. Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. Olfactory oscillations: The what, how and what for. Trends Neurosci. 2009; 32(4): 207-214. doi: 10.1016/j.tins.2008.11.008
29. Malvaut S, Saghatelyan A. The role of adult-born neurons in the constantly changing olfactory bulb network. Neural Plasticity. 2016; 1614329. doi: 10.1155/2016/1614329
30. Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AJ. Two distinct channels of olfactory bulb output. Neuron. 2012; 75(2): 320-329. doi: 10.1016/j.neuron.2012.05.017
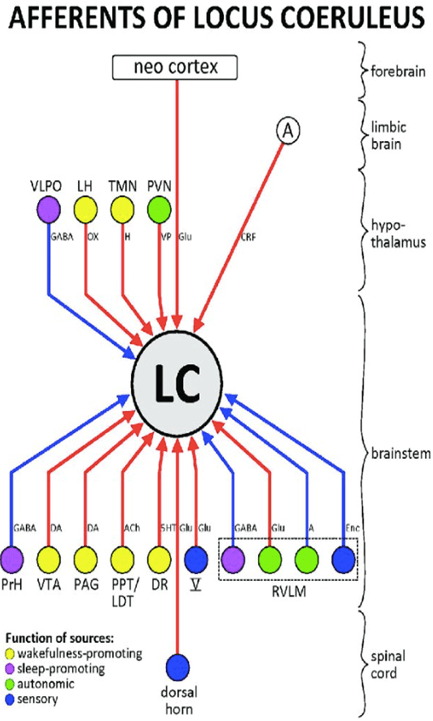
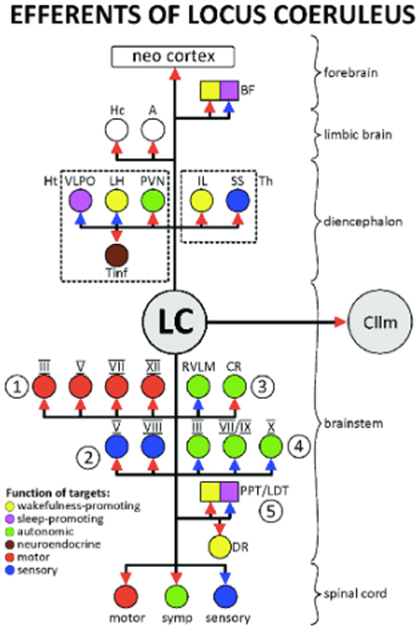
31. Schwarz LA, Luo L. Organization of the locus coeruleus-norepinephrine system. Curr. Biol. 2015; 25: R1051-R1056. doi: 10.1016/j.cub.2015.09.039
32. Carlson NR, Birkett MA. Physiology of Behavior. New Jersey, USA: Pearson Education, Inc. 2017; 559-576.
33. Vietl D. Interoception. Biological Psychology. 1996; 42(1-2): 1-27. doi: 10.1016/0301-0511(95)05144-9
34. Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005; 8(4): 519-526. doi: 10.1038/nn1421
35. Gilzenrat MS, Nieuwenhuis S, Jepma M, Cohen JD. Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cogn Affect Behav Neurosci. 2010; 10(2): 252-269. doi: 10.3758/CABN.10.2.252
36. Thorell LH, Wolfersdorf M, Straub R, et al. Electrodermal hyporeactivity as a trait marker for suicidal propensity in uni- and bipolar depression. J Psychiatr Res. 2013; 47(12): 1925-1931. doi: 10.1016/j.jpsychires.2013.08.017
37. Sarchiapone M, Gramaglia C, Iossue, et al. The association between electrodermal activity (EDA), depression and suicidal behaviour: A systematic review and narrative synthesis. BMC Psychiatry. 2018; 18(1): 22. doi: 10.1186/s12888-017-1551-4
38. Moretta T, Buodo G. Autonomic stress reactivity and craving in individuals with problematic internet use. PLoS One. 2018; 13(1): e0190951. doi: 10.1371/journal.pone.0190951
39. Wang P, Gao Y, Isen J, Tuvblad C, Raine A, Baker LA. Genetic covariance between psychopathic traits and anticipatory skin conductance responses to threat: Evidence for a potential endophenotype. Dev Psychopathol. 2015; 27(4 Pt 1): 1313- 1322. doi: 10.1017/S0954579414001424
40. Sudol K, Mann JJ. Biomarkers of suicide attempt behavior: Towards a biological model of risk. Curr Psychiatry Rep. 2017; 19(6): 31. doi: 10.1007/s11920-017-0781-y
41. Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007; 28(5): 409-423. doi: 10.1002/hbm.20291
42. Suominen K, Isometsä E, Martunnen M, Ostamo A, Lönnqvist J. Health care contacts before and after attempted suicide among adolescent and young adult versus older suicide attempters. Psychol Med. 2004; 34(2): 313-321. doi: 10.1017/s0033291703008882
43. Klebold S, Soloman A. A Mother’s Reckoning: Living in the Aftermath of Tragedy. New York, USA: Broadway Books. 2016.
44. Combi C, Keravnou-Papailiou E, Shahar Y. Temporal Information Systems in Medicine. New York, USA: Springer. 2010; 249-297.
45. Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006; 7(6): 477-484. doi: 10.1038/nrn1909
46. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015; 9:37. doi: 10.3389/fnins.2015.00037
47. Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiology of learning and memory. 2011; 96(2): 378-384. doi: 10.1016/j.nlm.2011.06.013
48. van Wingen GA, van Broekhoven F, Verkes RJ, et al. Progesterone selectively increases amygdala reactivity in women. Mol Psychiatry. 2008; 13(3): 325-333. doi: 10.1038/sj.mp.4002030
49. Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 2014; 111(2): 823-828. doi: 10.1073/pnas.1316909110
50. Bixo M, Johansson M, Timby E, Michalski L, Bäckström T. Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J Neuroendocrinol. 2018; 30(2). doi: 10.1111/jne.12553
51. Karpinski M, Mattina G, Steiner M. Effect of gonadal hormones on neurotransmitters implicated in the pathophysiology of obsessive-compulsive disorder: A critical review. Neuroendocrinology. 2017; 105(1): 1-16. doi: 10.1159/000453664
52. Altemus M, Sarvaiya, N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014; 35(3): 320-330. doi: 10.1016/j.yfrne.2014.05.004
53. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009; 48(3): 143-157. doi: 10.2165/00003088-200948030-00001
54. Miller MA. Gender-Based Differences in the toxicity of pharmaceuticals—The food and drug administration’s perspective. Int J Toxicol. 200; 20(3): 149-152. doi: 10.1080/109158101317097728
55. Reisz C. Recognizing off target drug effects at the gut and brain. Neurosci Commun. 2016; e1315. doi: 10.14800/nc.1315
56. Ropper AH, Brown RH. Chapter 4. Abnormalities of Movement and Posture Caused by Disease of the Basal Ganglia. Adam and Victor’s Principles of Neurology. New York, USA: McGraw Hill. 2005; 55-70.
57. Ropper AH, Brown RH. Chapter 43. Disorders of the Nervous System due to Drugs, Toxins and other Chemical Agents. Adam and Victor’s Principles of Neurology. New York, USA: McGraw Hill. 2005; 1016-1045.
58. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events: Implications for prevention. JAMA. 1995; 274(1): 29-34.
59. Weingart SN, Gandhi TK, Seger AC, et al. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005; 165(2): 234-240. doi: 10.1001/archinte.165.2.234
60. Qato DM, Ozenberger K, Olfson M. Prevalence of prescription medications with depression as a potential adverse effect among adults in the United States. JAMA. 2018; 319(22): 2289-2298. doi: 10.1001/jama.2018.6741
61. Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: An evaluation of 7 medical areas. JAMA. 2001; 285(4): 437-443. doi: 10.1001/jama.285.4.437
62. de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013; 17(5): 657-669. doi: 10.1016/j.cmet.2013.03.013
63. Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990; 38(9): 1277-1287. doi: 10.1177/38.9.1974900
64. Grall-Bronnec M, Victorri-Vigneau C, Donnio Y, et al. Dopamine agonists and impulse control disorders: A complex association. Drug Saf. 2018; 41(1): 19-75. doi: 10.1007/s40264-017-0590-6
65. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017; 17(1): 230. doi: 10.1186/s12877-017-0621-2
66. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010; 167(7): 748-751. doi: 10.1176/appi.ajp.2010.09091379
67. Lilienfeld S, Treadway M. Clashing diagnostic approaches: DSM-ICD Versus RDoC. Annu Rev Clin Psychol. 2016; 12: 435-463. doi: 10.1146/annurev-clinpsy-021815-093122
68. Szabadi E. Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol. 2013; 27(8): 659-693. doi: 10.1177/0269881113490326