INTRODUCTION
Spondyloarthritis (SpA) is a group of chronic inflammatory diseases with involvement mainly of the axial skeleton and peripheral joints. The enthesis is one of the target structures, and its inflammation, known as enthesitis, could go unnoticed.
The enthesis is considered the target structure of inflammation in SpA. Enthesitis, or inflammation of the enthesis, is characterized by tendon or ligament thickening and insertion site edema and causes bone erosion and new bone formation. This new bone formation developed due to enthesitis is called an enthesophyte in peripheral joints and a syndesmophyte in the spinal column. These enthesophytes can be seen on conventional radiography, especially in the calcaneus (Achilles tendon and plantar fascia).1 However, one of the significant limitations of conventional radiography is it’s poor ability to show soft tissue inflammation, which is common in enthesitis. Therefore, more sensitive methods such as ultrasound and magnetic resonance imaging (MRI) are often used. Both ultrasound and MRI can detect inflammatory areas in soft tissues. In addition, ultrasound has a series of technical advantages: it does not use radiation, it is relatively cheap, reproducible, and well accepted by patients and explorers, and it also has clinical benefits by offering an image in real-time and the possibility of evaluating multiple locations during the same ultrasound examination.2 The prevalence of enthesitis in SpA is not easy to determine due to its frequent subclinical involvement and the diagnostic difficulty involved in its clinical examination due to the absence of visible inflammatory signs. Due to this difficulty in the clinical evaluation of enthesitis, imaging techniques have potential use in its objective assessment.3
Ultrasound of the enthesis can be performed in a targeted manner on a specific enthesis according to the area referred to by the patient as painful in the anamnesis, or a more global assessment can be made by studying several entheses. For the study of various entheses, we described different ultrasound assessment indices. The Madrid Sonographic Enthesitis Index (MASEI) is the most complete to date and the only one based on the Outcome Measures in Rheumatologic Clinical Trials (OMERACT) definition of enthesopathy, evaluating 6 enthesis sites on each side of the body: the plantar fascia at its insertion with the lower pole of the upper calcaneus pole of the patella with the enthesis of the quadriceps tendon, the inferior pole of the patella with the proximal enthesis of the patellar tendon, the tuberosity of the tibia with the distal enthesis of the patellar tendon, the superior pole of the calcaneus with the enthesis of the Achilles tendon, and the enthesis of the tricipital fascia at its insertion with the olecranon tuberosity. In these sites, the tendon or ligament structure is thickening and causes the presence of bone erosions or calcifications at the enthesis site, the presence of a power-Doppler signal, and the presence of retrocalcaneal or infrapatellar bursitis. The MASEI showed high sensitivity (83.3%) and specificity (82.8%) in the diagnosis of SpA in patients with a score greater than or equal to 18.4
Therefore, the objective of our study is to predict the MASEI scores in patients with SpA based on regression models created by machine learning.
METHODS
Study Design
This is an observational, descriptive, and cross-sectional study. A retrospective review of a database of patients with SpA was under consideration.
Patients
Patients with SpA, according to Assessment of SpA International Society (ASAS) 2009 criteria, underwent musculoskeletal ultrasound using the MASEI and were treated in our clinics from May 2021 to September 2021.
Variables
For each patient, we have recorded 31 categorical and numerical variables grouped into five categories: descriptive, primary clinical manifestation, therapeutic options, inflammatory activity, and finally, the objective variable, the MASEI levels.
The descriptive variables are sex and age, the subtype of SpA (including psoriatic arthritis by classification of psoriatic arthritis (CASPAR) criteria), family history, time of evolution, and the presence of the histocompatibility complex human leukocyte antigens-B27 (HLA-B27).
As far as clinical manifestation is concerned, we assessed the presence of axial involvement, peripheral arthritis/ synovitis, enthesitis, dactylitis, uveitis, psoriasis, and inflammatory bowel disease, as well as imaging findings if sacroiliitis was evident based on the New York criteria, syndesmophytes, and bone edema as detected by MRI.
This study also takes into account the therapeutic options that the patient was given: corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), synthetic disease-modifying anti-rheumatic drugs (methotrexate and sulfasalazine), biologic drugs such as anti-tumor necrosis factor-alpha (TNF-α) (etanercept, adalimumab, golimumab, infliximab, and certolizumab), anti-interleukin (IL)-17, and anti-IL-12/23. We used two (yes/no) categorical variables to indicate if the patient received calciumvitamin D or vitamin D in the pre-treatment or post-treatment.
The inflammatory activity was assessed using ankylosing spondylitis disease activity score with C-reactive protein (ASDAS-CRP), which includes subjective variables such as questions about spinal pain, the patient’s global assessment, peripheral pain, swelling, or duration of stiffness, in addition to an objective variable of inflammation such as CRP or erythrocyte sedimentation rate (ESR). It is defined as an inactive disease when the score is <1.3, moderate activity is 1.3-2.1, high activity is 2.1-3.5, and very high activity is >3.5. On the other hand, we included bath ankylosing spondylitis diseases activity index (BASDAI) for patients with axial SpA using an easily applicable numerical rating scale of six questions concerning the disease: tiredness or fatigue, axial involvement, peripheral involvement, enthesopathy, and morning stiffness (2 questions). The range was 0 to 10, and the disease was considered active at a score higher than 4. Moreover, the disease activity for psoriatic arthritis (DAPSA) index was used for patients with psoriatic arthritis. It was calculated by linearly adding five variables: (1) number of swollen joints; (2) number of tender joints; (3) pain measured using a 0-10 visual numeric scale (VNS); (4) patient global assessment using a 0-10 VNS; and (5) CRP (mg/dL).
Finally, as a variable of interest for our study, the MASEI index evaluates the structure and thickness of the tendon, the presence of calcifications or enthesophytes, erosions in the bone, regional bursitis, and the presence of power doppler signal in the entheses in the following entheses: insertion of the quadriceps tendon in the superior pole of the patella, insertion of the patellar ligament at the inferior pole of the patella, patellar ligament attachment at the tibial tuberosity, Achilles tendon attachment at the calcaneus, plantar fascia attachment at the calcaneus, and triceps tendon attachment at the olecranon. The MASEI is an ultrasound index of entheses that is quickly performed and has good reproducibility since it assesses only six bilateral entheses, which are the most important in patients with SpA.
US Image Acquisition
An experienced rheumatologist performed ultrasound scans of the areas included in the MASEI on 24 consecutive patients who attended the rheumatology department of the university hospital. SpA patients fulfilled ASAS axial classification criteria8,9 and SpA patients fulfilled CASPAR criteria.10 The MASEI score systematically explored six bilateral enthesis locations, including the proximal plantar fascia, distal Achilles tendon, distal and proximal patellar ligaments, distal quadriceps, and brachial triceps tendons. Enthesis thickness, structure, calcification/bone proliferation, erosion, bursa, and power Doppler signal in the cortical bone profile, tendon, and bursa were scored (Table 1). A MyLab™ Twice (Esaote, Genoa, Italy) with a multifrequency linear array transducer (4-13 MHz) was used. The pulse repetition frequency was adjusted to 750 Hz. The color gain was set just below the color noise level. Each evaluation was performed from standardized positions. The triceps tendon evaluations were performed with the patient seated in front, arms in internal rotation, elbows flexed, and hands on a pillow on the thigh. The common extensor and flexor tendon evaluations were performed while patients’ arms were in a neutral position, elbows were flexed 90°, and hands were supine. The quadriceps, proximal and distal patella tendon, and anterior tibialis tendon were examined while patients were lying supine, knees were flexed 60°, and ankles were placed on the bed. The Achilles tendon evaluations were done while the patients were lying prone; the ankles were in a neutral position.11
| Table 1. Statistics of the Estimated Coefficients Parameters of the Linear Regression Model |
|
Estimate
|
SE |
tStat |
p value
|
| Intercept |
-4.0179
|
4.1657 |
-0.9645 |
0.3458
|
| ASDAS |
4.8388
|
1.9598 |
2.4690 |
0.0222
|
| Entersofites |
2.7527
|
0.7455 |
3.6922 |
0.0014
|
Statistical Analysis
In this paper, the dataset D={X,y} are the following: the endogenous variable y is the MASEI, and the variables X={xi} were selected using mutual information and F-tests that capture not only the variables most correlated with y but also those that can be part of a more complex model. Finally, we obtained the best machine learning model in terms of prediction by estimating the generalization error ‘e’ via 5-fold cross-validation to avoid overfitting, and we performed a hyper-parameter search via Bayesian optimization.
The mutual information test measures the difference between the joint distribution p(xi, y) and the product of marginal distributions p(xi) and p(y) using the Kullback-Leibler divergence function. We computed this test by estimating 1000 times the probability distributions and taking the mean of the results using the Scikit-learn library and the mutual information regressor. In addition, to obtain a complete perspective on the problem of choosing predictor variables, we also performed classical F-tests, computing the p values of the hypothesis that y and the predictor variables xi are drawn from populations with the same mean against the alternative hypothesis that the population means are not all the same, using the function fsrftest of Matlab.
Our regression models were obtained from the Matlab Regression Learner application that performs Bayesian optimization in the space of Machine Learning models on a total of 24 different methods grouped into neural networks, Gaussian Process Regression (GPR), Ensembles of trees, support vector machines (SVM), regression trees, and Linear regression models.
RESULTS
We analyzed twenty-four patients with SpA (with a mean age of 50.50±10.63-years), 8 women and 16 men. Regarding the clinical profile of the selected patients, they presented the following features: 5 patients with radiographic axial SpA, 4 non-radiographic axial SpA, 10 psoriatic arthritis, 2 SpA associated with inflammatory bowel disease, 2 patients with reactive arthritis, and finally, 1 patient with undifferentiated peripheral SpA. Twelve (12) had axial involvement, 18 had peripheral arthritis, 17 had clinical enthesitis, 10 had psoriasis, 11 had dactylitis, zero had uveitis, and 2 had inflammatory bowel disease. Regarding the treatment of our patients, 12 were treated with conventional Disease-Modifying Anti-Rheumatic Drugs (DMARDs) and 10 with biological DMARDs; of these last, 9 were treated with anti-TNF-α and 1 with anti-IL-17. In addition, 13 (54.16%) were on corticosteroids at some point, and 23 were on NSAIDs (95.83%).
According to the F-tests, the most important variables to explain the MASEI were activity, erosion regions, corticosteroids, enthesophyte regions, ASDAS, and the male sex (Figure 1). On the other hand, the mutual information test established almost the same variables in the feature selection, but in a different order: ASDAS, activity, enthesophyte regions, corticosteroids, and male sex (Figure 2).
Figure 1. Pareto Chart of the F-tests of MASEI Index with the Rest of the Variables
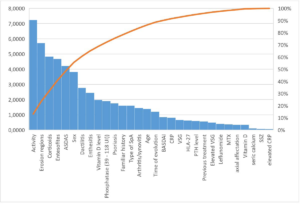
Figure 2. Pareto Chart of the Mutual Information test of MASEI Index with the Rest of the Variables
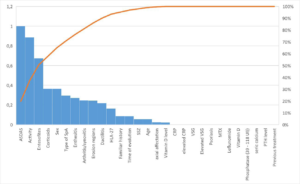
Considering the previous variables, the best regression model for explaining the MASEI was an SVM with a Gaussian kernel function. The model employs the following variables: Activity, corticosteroids, enthesophyte regions, ASDAS, and male sex. In this case, the variable erosion regions were not significant. The SVM model obtained the lowest root mean square error (RMSE) in validation (6.14); besides, it also has the most significant R-squared error in validation (0.81). Other technical features of this model can be consulted in the left part of Table 2.
| Table 2. Technical Features of the SVM Model and the Linear Regression Model |
|
Support Vector Machine
|
Linear Regression Model
|
|
Training Results
|
| RMSE (validation) |
6.1382
|
8.9983
|
| R-Squared (validation) |
0.81
|
0.60
|
| MSE (validation) |
37.677
|
80.97
|
| MAE (validation) |
4.5485
|
7.0781
|
| Prediction speed |
-940 obs/sec
|
-880 obs/sec
|
| Training time |
0.79655 sec
|
1.4912 sec
|
|
Model Type
|
|
Preset Medium Gaussian SVM
|
Preset: Linear
|
|
Kernel function: Gaussian
|
Terms: Linear
|
|
Kernel scale: 2.4
|
Robust option: Off
|
|
Box constraint: Automatic
|
|
|
Epsilon: Automatic
|
|
|
Standardize data: true
|
|
|
Optimizer Options
|
|
|
Hyperparameter options disabled
|
|
Feature Selection |
|
|
|
Used features, before PCA: ASDAS, Entesofites
|
|
|
PCA disabled
|
Since the previous model is a black box, we also computed the best explicit model, a linear regression, which, although it has a bigger RMSE in validation (8.99) and a worse R-squared error in validation (0.60) than the former one, is an entirely explicit model, given by: MASEI=-4.02+2,75 Enthesofites+4.838 ASDAS.
The p values of the variables are recorded in Table 1; both are significant (p<0.05). Other technical features of this model can be consulted in the right part of Table 2.
The differences in the accuracy of both models can be observed in Figure 3. The SVM model, which works better with high dimensionality, avoids more extreme cases. That is why real data is more concentrated around the diagonal in the SVM model.
Figure 3. True vs Predicted Response of the SVM Model and the Linear Regression Model
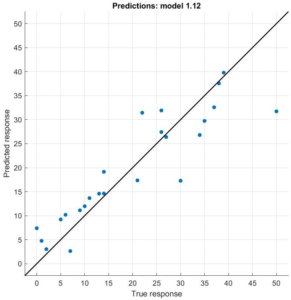
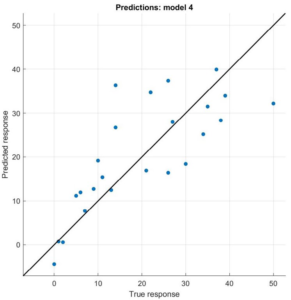
DISCUSSION
Several publications point out the relationship between MASEI and inflammatory activity in SpA patients. The works published to date attempt to quantify enthesis involvement and establish a relationship or association with the composite indices of inflammatory activity, but have not found conclusive or significant results. However, they show that the higher the MASEI, the more activity of the disease in patients with SpA. As in the recent publication of Macía in 2021, although it focused solely on patients with psoriatic arthritis, the 27 patients studied showed how tendon thickening and enthesophytes (such as enthesis lesions) were more frequently associated with peripheral radiographic damage and sacroiliac, and therefore, with more significant disease activity and worse assessment by the patient.12 In 2015, El Miedany’s group published a study in which they identified potential predictors of early joint structural damage in psoriatic arthritis using another enthesis assessment index, known as Glasgow Ultrasound Enthesitis Scoring System (GUESS), and found a greater probability of structural progression over one year related to Doppler signal in the entheses and total GUESS score.13
In our work, we observed the importance of activity disease, corticosteroids, enthesophyte regions, ASDAS, and male sex to explain the MASEI index in an SVM model, which optimizes the predictions in a space of machine learning models. These results are similar to the publications made by other authors, in which it is affirmed that a patient with greater activity of the disease presents a higher MASEI12,13 and also with Doppler activity in the enthesis, as in the publication carried out by Falcao in 2015, evaluating only the Achilles as the only enthesitic region. This group is able to show that baseline ESR and CRP are higher in patients with a Doppler signal at the enthesis and that higher baseline ESR, CRP, and ASDAS predict a higher Doppler signal.14 The other regression model, the linear one, only considers the variables enthesophyte regions and ASDAS; both are significant, with p values inferior to 0.05.
To highlight as a limitation of our work that a good attribute in the search of the estimator f(X) is that our predictions have low bias and variance: if bias is high, there is underfitting, and our model is not complex enough to approximate reality; if the variance is high, there is overfitting, and our model is fixing noise and misses the general trend.
CONCLUSION
The Support Vector Machine model is often the best machine learning model when the number of variables is high, and the number of cases is low, as was the case in our ultrasonographic study of SpA patients.
ETHICAL APPROVAL INFORMATION
The Clinical Research Ethics Committee of the General University Hospital of Ciudad Real approved our research on June 29, 2021 (Act 07/2021). In addition, we obtained the patient’s written informed consent to publish the material, confirming that all experiments were performed following all the relevant guidelines and regulations.
ACKNOWLEDGEMENTS
We would like to thank the rheumatology department of Ciudad Real for their support and dedication. Without them and their patients, this study would not have been possible. Special mention to Dr. Calvo Pascual from the University of Comillas for his time and analysis of the study.
FUNDING
All authors declare that they have no source of funding for this work.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.









