BACKGROUND
Patients in the hospital are at an increased risk of developing a deep vein thrombosis or pulmonary embolism. Some risk factors including recent surgery, vascular injury, or hypercoagulability increase a patient’s risk for a venous thromboembolism (VTE); however, these events can occur in patients without risk factors, as well. The reported rate of VTE in medically ill patients is 10-26%.1,2 Due to this risk, it is important for all acutely ill, hospitalized patients to receive pharmacologic or mechanical prophylaxis, to avoid a preventable VTE from occurring. Venous thromboembolism prophylaxis is considered a core measure by The Joint Commission and Centers for Medicare and Medicaid Services for all patients older than 18 years of age and with a hospitalization length of stay at least 2 days.3,4 Pharmacologic prophylaxis has demonstrated the potential to reduce VTE events by 50-65% in acutely ill patients with a favorable safety profile.5
Patients with cystic fibrosis (CF) are frequently hospitalized for intravenous antibiotics due to persistent pulmonary infections and difficulty in eradicating pulmonary bacterial colonization. These patients, similar to other acutely ill medical patients, have an 8-fold increased risk of VTE.3 Some common risk factors among acute medically ill patients and those with CF include decreased respiratory function and decreased mobility.6,7,8 Additional VTE risk factors identified for patients with CF include the presence of central venous catheters, colonization with Burkholderia cepacia complex (BCC), ongoing inflammation, liver dysfunction, platelet hyper-reactivity and vitamin K deficiency.9,10,11,12,13 Several studies have evaluated the rates of VTE in patients with CF, but these studies vary in design and length, ranging from 2-13 years. The rate of VTE in adults and children with CF is reported between 3.5% and 16.1%.11,14,15,16,17
Venous thromboembolism in patients with CF has the potential to increase morbidity, mortality and health care costs. Development of a pulmonary embolism will further decrease an already limited pulmonary reserve, as well as introduce potential complications from long-term anticoagulation, such as hemoptysis or other bleeding and the potential need for therapeutic drug monitoring. This study characterizes refusal rates of pharmacologic VTE prophylaxis in adult patients with CF at a large CF-accredited center. Furthermore, the study assesses the resulting incidence of VTE of patients during their admission as well as within 30 days of hospital discharge during a two-year study period.
METHODS
The study is a retrospective, single-center medical record cohort review of adult patients (≥18 years) with CF (based on International Classification of Diseases, Ninth Revision (ICD-9) billing code 277) admitted to the hospital between September 1, 2013 and August 30, 2015. Patients who were receiving therapeutic anticoagulation, who were pregnant, had an active VTE, or who had previously received a lung transplant were excluded. The Institutional Review Boards at both Mercer University and Emory University approved the study procedures.
Medical and laboratory data were collected from the electronic medical record. Patient demographics and clinical data including age, sex, height, weight, serum creatinine at baseline, percent predicted forced expiratory volume in the first second (ppFEV1) at admission, ppFEV1 at baseline, liver function tests, length of stay, past medical history, and history of VTE were collected. Creatinine clearance and body mass index (BMI) were calculated from the collected data.
The primary endpoint for the study was to determine the rate of refusal of pharmacologic VTE prophylaxis in adult patients with cystic fibrosis. Secondary endpoints include the incidence of VTE during hospitalization, the incidence of VTE within 30 days following the date of discharge, the combined incidence of VTE during hospitalization and within 30 days following the date of discharge, the difference in refusal rate between pharmacologic agents, a comparison between patients who refused prophylaxis and those who did not refuse prophylaxis, and the incidence of VTE in patients with BCC.
Patients were divided into two categories: refused prophylaxis and did not refuse prophylaxis. Patients were defined as refusing their pharmacologic prophylaxis if 50% or more of the scheduled administrations were refused during the first 48 hours of admission.
Statistical analysis was performed with IBM Statistical Package for the Social Sciences (SPSS) Software. Descriptive statistics were calculated for baseline characteristics along with primary and secondary endpoints. Chi-square tests were used to assess difference in categorical variables and student t-tests were used to assess the differences in continuous variables. An α of ≤0.05 was considered to be statistically significant.
RESULTS
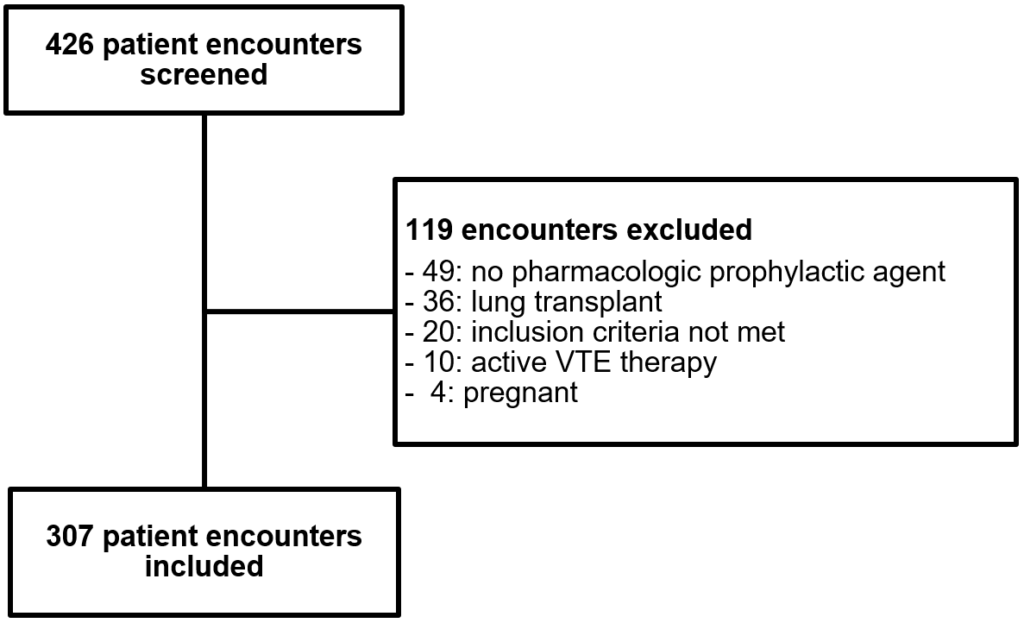
Four hundred and twenty-six patient encounters were identified based on the ICD-9 billing code for CF and admission to the hospital during the defined study period. Of these, 307 patient encounters of 144 unique patients were included in the study (Figure 1). One hundred and nineteen patient encounters were excluded because they did not have a pharmacologic prophylaxis agent ordered (n=49), had previously received a lung transplant (n=36), were receiving treatment for VTE (n=10), were pregnant (n=4) or did not meet other inclusion criteria (n=20).
Figure 1: Patient Enrollment
Characteristics of the patients included in the study are described in Table 1. Patients were approximately 28 years of age with a body mass index of 21 kg/m2. The mean ppFEV1 at admission, an important measure of pulmonary function, was just under 48%, which was reduced from the patients’ mean baseline ppFEV1 of around 60%. Unfractionated heparin 5000 units administered subcutaneously every eight hours was the most common regimen used for VTE prophylaxis, ordered in 62.5% of patients.
| Table 1: Characteristics of the Study Population (n=307). |
| Characteristic |
Study Populationa |
| Age [years] |
28.4±8.9 |
| Male [%] |
50 |
| Height [cm] |
166.1±10.2 |
| Weight [kg] |
58.4±13.7 |
| Body Mass Index [kg/m2] |
21.0±3.8 |
| Serum Creatinine [mg/dL] |
0.9±0.3 |
| Creatinine Clearance [mL/min] |
105.6±32.5 |
| ppFEV1 Admissionb |
47.9±22.5 |
| ppFEV1 Baselineb |
59.9±24.3 |
| Length of Stay |
4.9±3.3 |
| History of VTE [%] |
3.3 |
aMean ± standard deviation.
bppFEV1: Percent predicted forced expiratory volume in the first second. |
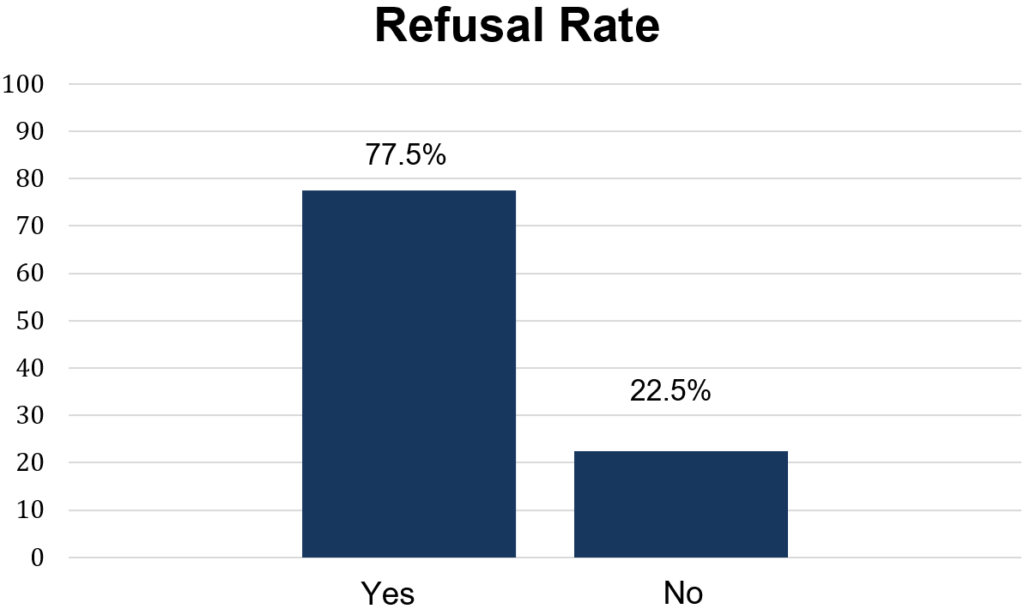
Pharmacologic VTE prophylaxis was refused during 238 (77.5%) patient encounters. Over 22% of patients had pharmacologic prophylaxis administered during their encounters (Figure 2). No differences were seen in the rates of refusal based on pharmacologic agent or dosing interval. Unfractionated heparin was refused by 77.8% of patients and enoxaparin was refused by 75.0% of patients (p-value = 0.84).
Figure 2: Primary Outcome: Refusal Rate of Pharmacologic VTE Prophylaxis.
The characteristics of the patients who refused pharmacologic prophylaxis were compared with patients who did not refuse and are shown in Table 2. Patients who were younger (p=0.003), weighed less (p=0.017) and had a lower BMI (p=0.008) were significantly more likely to refuse pharmacologic prophylaxis compared to those who did not refuse.
| Table 2: Comparison of Baseline Characteristics for Patients who Refused and did not Refuse Pharmacologic Prophylaxis. |
| Characteristic |
Refused |
Did not refuse |
p-value |
| Age [years]a |
27.5±8.2 |
31.6±10.4 |
0.003 |
| Sexb
Male
Female |
114 (47.9)
124 (52.1)
|
39 (56.5)
30 (43.5) |
0.221 |
| Height [cm]a |
165.9±10.2 |
166.6±10.1 |
0.648 |
| Weight [kg]a |
57.2±12.6 |
62.4±16.4 |
0.017 |
| Body Mass Index [kg/m2]a |
20.7±3.4 |
22.3±4.8 |
0.008 |
| Serum Creatinine [mg/dL]a |
0.8±0.3 |
0.9±0.4 |
0.222 |
| Creatinine Clearance [mL/min]a |
105.5±32.3 |
105.7±33.6 |
0.973 |
| ppFEV1 Admissiona,c |
47.3±21.6 |
50.3±25.5 |
0.404 |
| ppFEV1 Baselinea,c |
58.8±23.7 |
63.9±25.9 |
0.123 |
| Length of Stay [days]a |
4.9±3.1 |
5.3±4.2 |
0.410 |
| History of VTEb
No
Yes |
232 (97.5)
6 (2.5)
|
65 (94.2%)
4 (5.8%) |
0.240 |
| VTE Orderb
Heparin 5000 units q12h
Heparin 5000 units q8h
Enoxaparin 30 mg daily
Enoxaparin 40mg daily |
8 (3.4)
152 (63.9)
9 (3.8)
69 (29.0)
|
3 (4.3)
40 (58.0)
3 (4.3)
23 (33.3) |
0.844 |
aMean±standard deviation.
bn(%).
cppFEV1: Percent predicted forced expiratory volume in the first second. |
A VTE event occurred in five patients total with three events during the hospitalization and two different patients within 30 days of discharge. The combined incidence of VTE per hospitalization was 1.63%. Three of the patients developed a pulmonary embolism and the two patients developed an upper extremity deep vein thrombosis. All of the patients who developed a VTE refused their pharmacologic prophylaxis agent, with 80% of these patient encounters having an order for enoxaparin 40 mg subcutaneous every 24 hours. No differences were observed between patients who refused and did not refuse prophylaxis with respect to developing a VTE during hospitalization, within 30 days or as a combined incidence, likely due to the small number of VTE events. None of the patients who developed a VTE during the study period had a microbiological history of colonization with BCC.
DISCUSSION
Refusal of pharmacologic VTE prophylaxis is high among adult patients with CF and occurred in three-fourths of the study patients. Refusal was similar between all pharmacologic agents and was not dependent on the number of administrations per day. Refusal of pharmacologic prophylaxis was chosen as the primary endpoint since there was a concern at the study institution about an increased number of VTE events and no previous available literature characterizing the role of pharmacologic prophylaxis refusal in this patient population.
The combined incidence of VTE observed in this study (1.63%) was lower than previously reported. The previous studies that have quantified the rates of VTE in patients with CF have varied in duration from 2 years to 13 years14,15,16 with two of the studies being prospective observations.11,17 The three retrospective reviews that are most similar to our patient population included adults with CF who had central venous catheters and reported VTE rates from 3.5% to 16.1%.14,15,16 Nash et al also evaluated the rate of VTE in patients with a positive culture for BCC. The rate of VTE was significantly higher at 20.9% (p=0.02) compared to those with negative cultures.16
Two studies reported their findings from prospective studies evaluating both children and adult patients with CF, all with central venous catheters and found the rate of VTE to be 3.7-6.6%.11,17 Similarly, Raffini et al reported a high incidence of VTE (27%) in patients with a history of BCC.11
This study evaluated patient encounters, as many patients were admitted to the hospital multiple times during the study period and had the opportunity to refuse or accept pharmacologic prophylaxis at each admission. Of the combined incidence of VTE observed, five different patients developed a venous thromboembolism. It could be argued the combined incidence based on individual patients (n=144) was higher at 3.8%; however, this is still on the lower end of the rates reported previously.
The statistical findings from the post-hoc analysis did identify younger age, lower body weight, and lower body mass index as factors associated with increased refusal of pharmacologic prophylaxis. Younger patients might be more likely to refuse their prophylaxis as they may have had fewer hospital admissions and be less familiar with the purpose of VTE prophylaxis, particularly if they were more recently treated in a pediatric hospital where VTE prophylaxis is less routine, or they may be more ambulatory in the hospital. Additionally, patients with lower body weight or body mass index may have more severe disease and frequent hospital admissions, or be concerned about injection site pain due to their small body size. These associations may be due to chance and may not represent risk factors for refusal.
The findings of this study illustrate a high rate of VTE prophylaxis refusal with a low rate of resulting venous thromboembolism. Although, pharmacologic prophylaxis is more effective and the preferred strategy for preventing VTE, it may be reasonable to calculate the VTE risk for each patient to determine if pharmacologic prophylaxis is necessary and to educate high-risk patients about the benefits of VTE prophylaxis.
LIMITATIONS
This study is limited by its single-center, retrospective design; however, the large number of patient encounters included in this evaluation and the continuity of care between inpatient admissions and the outpatient clinic visits abrogates this limitation. The low number of VTE events is an additional limitation since the small numbers did not allow for comparisons between those who developed VTE versus those who did not. Third, a bias of inclusion can be considered a limitation, as patient encounters were included in the study and not individual patients. Patients were admitted multiple times during the two-year time period and are more likely to refuse their prophylactic agents if they have refused in the past. It is possible that although most of these patients follow-up at the CF clinic, some may have been admitted to other hospitals with VTE events or seen at other medical clinics. Lastly, the presence or type of central venous catheter was not assessed in this study.
CONCLUSIONS
The majority of adult patients with CF admitted to the hospital over a two-year period refused pharmacologic VTE prophylaxis with no difference in refusal between pharmacologic agents, and the combined incidence of VTE was low.
ACKNOWLEDGEMENTS
Kathryn M. Momary, PharmD, BCPS assisted with statistical analysis and is a Vice Chair and Associate Professor in the Department of Pharmacy Practice at Mercer University College of Pharmacy.
FINANCIAL SUPPORT
None.
CONFLICTS OF INTEREST
The author(s) declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
PRESENTATIONS
An abstract from this study was presented at the United Health Consortium poster session in New Orleans, LA on December 5, 2015, and the Southeastern Residency Conference in Athens, GA on April 28, 2016.







