INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is often associated with the metabolic syndrome1,2 and also with hypertension, hyperlipidemia, ischemic heart disease, cerebrovascular disease, and impaired glucose metabolism.3,4,5 Because of the relationship between OSAS and type 2 diabetes mellitus (T2DM), the effectiveness of continuous positive airway pressure (CPAP) therapy in patients with T2DM has been assessed in many trials, with Hemoblobin A1c (HbA1c) levels improving in some patients.6,7 However, the effects of prosthetic mandibular advancement (PMA) on blood glucose levels and insulin resistance remain unclear.
Continuous glucose monitoring system (CGMS) is a recently developed electronic system designed to continuously monitor subcutaneous glucose concentration in the interstitial fluid. CGMS is a powerful tool for T2DM control because it provides a detailed daily blood glucose profile.8
Here we assessed the immediate effect of PMA on glycemic control measured using CGMS in a T2DM patient with OSAS.
CASE REPORT
77-year-old Japanese woman with T2DM [height, 146.6 cm; weight, 64.4 kg; Body Mass Index (BMI), 30.0; Table 1] was admitted to Fukuoka University Hospital, Japan for attending a diabetes mellitus education program. Her Fasting Blood Sugar (FBS) and serum C-peptide levels were 152 mg/dL and 2.22 ng/mL, respectively, indicating a relatively maintained insulin secretory ability. The patient was started on a diet of 1400 kcal/day during her hospital stay. For 14 days, her FBS levels were well controlled; hence insulin therapy was discontinued and only the 1400 kcal/day diet was maintained.
Table 1: Clinical features of the patient with T2DM.
|
Factors
|
|
Age (year)
|
77
|
|
Gender
|
F |
|
Height (cm)
|
146.6 |
|
Weight(kg)
|
64.4
|
|
Body mass index (kg/m2 )
|
30.0
|
|
Blood pressure (mmHg)
|
125/81 |
| Fasting blood sugar (mg/dl) |
152
|
|
Hemoglobin A1c (NGSP)%
|
6.2 |
|
Low-density lipoprotein (mg/dl)
|
58 |
|
High-density lipoprotein (mg/dl)
|
38
|
|
Triglyceride (mg/dl)
|
271 |
|
Duration of diabetes (year)
|
2.0 |
|
Medicine for diabetes mellitus
|
Insulin therapy was finished
|
|
Other medicines
|
|
Hypertension
Antiplatelet
Hyperlipemia
Anemia
|
Olmesartan, Medoxomil,
Nifedipine, Aspirin,
Clopidogrel, Ethyl Icosapentate,
Pitavastatin Calcium, ferrous Citrate, Folic Acid
|
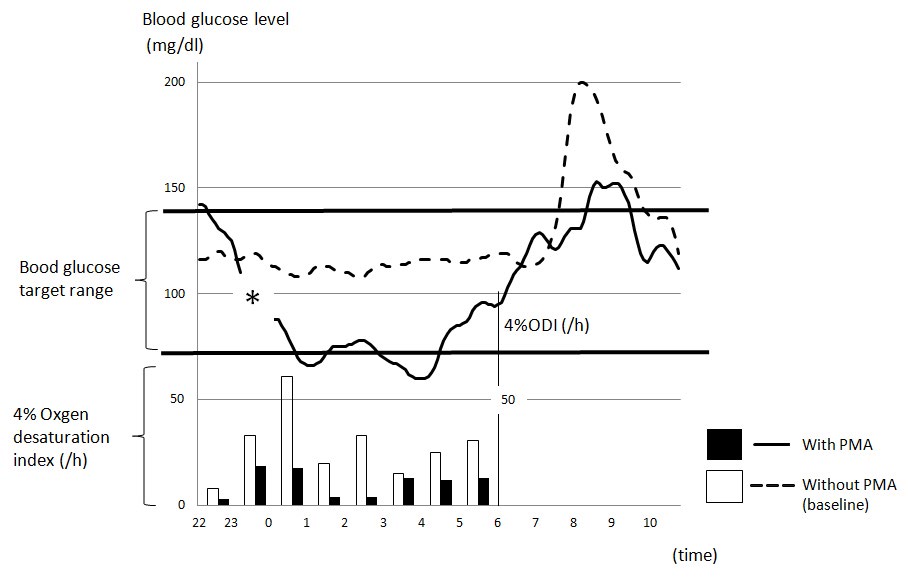
Subsequently, she achieved good glycemic control and did not require other medication, such as oral hypoglycemic agents. On the third hospital day, she was hypoxemic with peripheral oxygen saturation (SpO2) of 92% measured at rest while breathing room air; however, she did not have subjective symptoms of breathing difficulty. Spirometry testing demonstrated a Forced Vital Capacity (FVC) of 1880 mL (93.5%) and forced expiratory volume in 1 s to FVC ration of 69.2%. An obesity-associated decrease in the movement of the diaphragm possibly caused the SpO2 drop in the supine position. Because she was suspected to have OSAS, we investigated the degree of sleep apnea using a 2-channel (airflow and SpO2) portable sleep apnea monitor (LS-120/120S, Fukuda Denshi Co, Ltd, Tokyo, Japan). The SpO2 decreased and she was finally diagnosed with OSAS with a Respiratory Disturbance Index (RDI) of 39.3. Because she refused CPAP therapy, she was referred to the Department of Oral and Maxillofacial Surgery for PMA. She wore a customized PMA that advanced the mandible 7 mm forward for the evaluation of sleep apnea. During the evaluation, BS levels were monitored using CGMS (iPro2, Medtronic-MiniMed, Northridge, CA, USA), which records the interstitial glucose level every 5 min for up to 72 hr. The CGMS data were analyzed using the CareLink iPro2 software application (Medtronic) and corrected using self-monitoring of blood glucose four times daily. Interstitial glucose levels were monitored for 2 consecutive night: first night without PMA (baseline) and the second night with PMA (12 h of recording each night from 10 pm to 10 am). We obtained 152 measurements on the first night and 143 on the second night. CGMS data were missing for 45 min during the second night (asterisk in Figure1) because of poor contact with the glucose sensor.
PMA resulted in a marked reduction in the RDI from 39.3 to 12.8 PMA (Table 2). With PMA, we observed an increase in the minimum hemoglobin saturation from 78.0% to 87.0%. The mean glucose level (10 pm to 10 am) remarkably improved with PMA from 126.1±24.6 to 100.5±29.6 mg/dL (20.3% decrease, p<0.001; Figure 1). Furthermore, the number of episodes of oxygen desaturation of >4% below baseline during the night (10 pm to 6:30 am) decreased from 226/h to 86/h.
Table 2: Results of the portable sleep apnea monitor and CGMS.
|
Portable Sleep apnea monitor
|
Without PMA (Baseline) |
With PMA |
|
Total scoring time (min)
|
433 |
425 |
|
Respiratory disturbance index (/h)
|
39.3 |
12.8
|
|
Apnea/Hypopnea Episode
|
196/96 |
57/34
|
| Max Period of Apnea Lasting (sec) |
71 |
47
|
|
4% Oxgen desaturation index (times)
|
226 |
86
|
|
minSpO2 (%)
|
78 |
87
|
|
meanSpO2 (%)
|
94 |
94
|
|
CGMS
|
|
mean blood glucose (mg/dl) ±SD (min-max)
|
126.1±24.6 (108-200) |
100.5±29.6 (60-153)
|
Figure 1: Line and bar graphs showing changes in the mean glucose level using the CGMS during the night (10 pm to 10 am) and the number of episodes of oxygen desaturation of >4% below baseline /h during the night (10 pm to 6:60 am) respectively. *Glucose levels were not monitored from 11:15 pm to midnight because of difficulty with a sensor.
DISCUSSION
OSAS is characterized by repetitive episodes of upper airway obstruction occurring during sleep, generally associated with a decrease in blood oxygen saturation.9 Furthermore, OSAS is associated with insulin resistance and T2DM.10,11 Tamura, et al.11 reported that Impaired Glucose Tolerance (IGT) was observed in 60.5% patients with sleep apnea (30.2% with T2DM as well). Another study reported that T2DM was present in 30.1% patients with OSAS and 13.9 snorers; IGT was diagnosed in 20.0% patients with OSAS and 13.9% non-apneic snorers.12 In addition, the study reported that insulin sensitivity decreased when the severity of sleep apnea increased. Different studies have indicated that BMI is the major factor for insulin resistance in patients with OSAS.13,14 However, even after controlling for obesity and other confounding factors of insulin resistance, the apnea-hypopnea index and/or a minimum SpO2 were reported to be still associated with fasting insulin level and Homeostasis model assessment of insulin resistance (HOMAIR).13 Diabetic control is generally because of increase in insulin secretion, insulin sensitivity, or both. In our case, although the serum C-peptide level was normal, HOMA-IR was not performed because of the insulin therapy in the initial stage, thus impeding the estimation of the presence or absence of insulin resistance. Hence, the reason for the rapid FBS improvement during the night with PMA remains unclear.
Spiegel K, et al.15 assessed carbohydrate metabolism in 11 young men who had their sleep duration restricted to 4 h/ night for 6 nights. Glucose tolerance was lower in participants deprived of sleep than in those completely rested; in addition, evening cortisol levels were significantly elevated in the sleepdeprived participants.15 Another study found a decrease in oxyhemoglobin saturation, which was induced when the patients were awake, and suggested that this intermittent hypoxia was associated with a decrease in insulin sensitivity.16 In a recent study, after 2 nights of sleep fragmentation, decreased insulin sensitivity and glucose effectiveness was observed, i.e, the ability of glucose to mobilize itself independently in response to insulin had decreased.17 The duration of T2DM in our patient was only 2 years, and she had no subjective symptoms of OSAS, including daytime sleepiness or snoring. Obesity-induced desaturation and OSAS might be partly associated with the occurrence of T2DM in this patient.
A previous study evaluated the insulin resistance of 40 patients with OSAS before and after CPAP therapy based on hyperinsulinemic-euglycemic clamp studies, and found that insulin resistance improved after CPAP therapy, particularly in non-obese patients.18 Using a 72-h CGMS, Babu, et al.6 studied the changes in interstitial glucose levels and measured HbA1c levels in 25 patients with T2DM before and after CPAP therapy for OSAS. After CPAP therapy, they observed a significant decrease in both 1-h postprandial glucose and HbA1c levels after CPAP therapy.6 Furthermore, a significant correlation between decrease in HbA1c levels and the duration of CPAP therapy was observed in patients who used CPAP for more than 4 h/day. Our case showed a decrease in FBS levels similar to that found in studies using CPAP therapy.6,18 This result suggests that PMA may have an equal effect on blood glucose levels as CPAP therapy.
PMA prevents upper airway collapse in patients with OSAS. Recent American Academy of Sleep Medicine guidelines concluded that oral appliances are less effective than CPAP but are a reasonable alternative for patients with mild to moderate OSAS in specific situations.19,20To the best of our knowledge, the present case report is the first showing the impact of PMA on glycemic control assessed using CGMS.
Improvement of intermittent hypoxia by wearing PMA during the nights may have had a beneficial effect on glycemic control in our case. Any significant improvement of RDI and oxygen saturation levels achieved with PMA may have an immediate effect on blood glucose levels in a patient with T2DM. Because here we have reported only one such case, further investigations are required to confirm whether the beneficial effect of PMA can be observed in a large number of T2DM patients complicated with OSAS.
In conclusion, our T2DM patient with OSAS showed improvement in RDI and glucose levels after wearing a PMA during the night. This case suggests an immediate effect of the PMA on glycemic control. The results obtained using CGMS in this case, regarding the effect of PMA on glycemic control support the importance of adequate treatment in T2DM patients with OSAS. Further discussion of such benefits can help providers promote compliance in patients with T2DM.
CONFLICTS OF INTEREST
None.
CONSENT
The patient has provided written permission for publication of the case details.






