INTRODUCTION
Endometrial hyperplasia is considered the most common precursor of gynecological cancer diagnosed in women. The incidence of endometrial hyperplasia is indistinctly reported to be around 200,000 new cases per year in Western countries, this is caused by a disordered proliferation of the endometrial glands that are the result of unopposed estrogenic stimulation of endometrial tissue with compensatory progesterone deficiency. The average age of diagnosis is in postmenopausal women between the ages of 50-54-years.1
Endometrial hyperplasia the most common cause of abnormal uterine bleeding leading to endometrial cancer; if not treated in time, 10% of these patients’ histological results show endometrial hyperplasia.1,2 The main function of endometrial sampling in patients with abnormal uterine bleeding is to determine whether there are carcinomatous or premalignant lesions to decide the most appropriate management. The risk factors for developing endometrial cancer involved are obesity, estrogen therapy, diabetes, hypertension, polycystic ovarian syndrome, Lynch syndrome, treatment, and nulliparity.3
Risk of Progression
Endometrial hyperplasia represents a continuum of histologically distinct processes, starting from simple endometrial hyperplasia without atypia and then progress to complex endometrial hyperplasia with atypia, followed by well-differentiated endometrial carcinoma. The presence and severity of cytological atypia and architectural crowding are key factors defining the risk for progression to carcinoma. Simple hyperplasia shows the lowest risk of cancer progression. Among patients with atypical hyperplasia, postmenopausal status is associated with the highest risk of progression to adenocarcinoma. Complex hyperplasia has an intermediate risk of progression, which has been shown to regress in most of cases, while endometrial hyperplasia with cytological atypia is characterized as direct precancerous lesions and may carry a higher risk of progression to carcinoma.
According to the context of the aforementioned, endometrial anomalies are considered to be diagnostic challenges faced by radiologists and gynecologists who evaluate them. The ultrasonographic study is the most important imaging modality; however, magnetic resonance imaging can be used to correlate findings.3,4 To induce the regression of hyperplasia, treatment with progestogens with duration of 6-months should be considered; monitoring with biopsy at 6-month intervals is recommended. Surgery is indicated when medical treatment fails or when the patient refuses followup.4
CASE REPORT
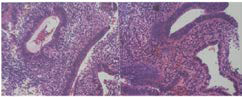
A 50-year-old woman from Sololá, Guatemala with no previous medical or surgical history, attends a gynecological evaluation for a history of abnormal uterine bleeding of three days of evolution, which is abundant without extenuating or exacerbating, the Date of the last menstruation was April 2020. The transvaginal ultrasound (Figures 1 and 2 ), showed a endometrium markedly thickened with multiple asymmetric cystic lesions diffused distribution. The thickness of endometrium was 18 mm, with an adequate endometrial-myometrial interface., It was reported as cystic endometrial hyper plasia, however, due to the age of the patient, a correlation with a histopathological study was recommended which reports simple endometrial hyperplasia without atipia (Figure 3). In the follow-up, the patient underwent surgery with another gynecologist.
Figure 1. Transvaginal Ultrasound, the Uterus it is Seem with Smooth Margins, with Multiple Miometrial Cysts. The Miometrial-Endometrial Interfacee are well Demonstrated
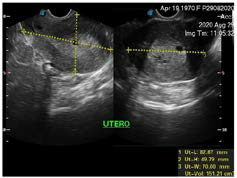
Figure 2. The Thickness of the Endometrium was 18.09 mm, Look at the Innumerable Cystic Lesions which are Asymmetrical in Size
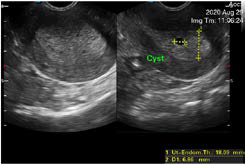
Figure 3. Biopsy, Demonstrates Hyper Plastic Endometrium without Atypic Cells. (WHO). Courtesy PATMED (Orlando Rodas Pernillo, and Eliza Hernandez MD pathologist)
DISCUSSION
Endometrial hyperplasia, which is defined as a disorderly proliferation of the endometrium, the result of estrogenic stimulation and progesterone insufficiency. The proliferating glands that could vary in shape and size show cytological atypia at this point; it is necessary to bear in mind that the endometrium can present a series of changes that can be considered normal as pathological at menarche, as well as in the prepubertal years, postmenopause and the first trimester of pregnancy.1 It is very important to understand that the appearance of the endometrium can be related to different factors, including age, the stage of the menstrual cycle, pregnancy status, and if you have undergone significant hormone replacement therapy or tamoxifen therapy in order to accurately diagnose.1,2
The diagnosis of endometrial hyperplasia without atypia is seen to occur in patients between the ages of 50-54-years, as is the case of the patient in the article, hyperplasia with atypia is more frequent in women between 60-64-years, its presentation being very rare in under 30.3 Most women have a clinical presentation of abnormal uterine bleeding, estimating in previous studies that endometrial hyperplasia represents 15% of all postmenopausal bleeding.4
Obese patients with chronic anovulatory dysfunction have a high-risk of endometrial hyperplasia as well as an increased risk of endometrial cancer.4,5
Recamier in 1850 first recognized endometrial hyperplasia. It was since then that an attempt had been made to classify endometrial hyperplasia in different ways, the importance of which is that the pathologist must use criteria and terminologies that clearly distinguish the clinicopathological characteristics.5 The attempt to reclassify the collected data has generated a vast lexicon for endometrial cancer perpetrators.5
The classification system most used today is the one based on Kurman et al6 a scheme that describes architectural characteristics and cytological atypia necessary to be able to identify the percussive lesions that define atypical endometrial hyperplasia. The classification of the World Health Organization 1994 (WHO 94) is described as follows (Table 1):
For a correct diagnosis of endometrial hyperplasia, it is necessary to determine the differential diagnosis criteria, since many characteristics of atypia can also be observed in menstrual irregularities since endometrial hyperplasia is one of the most frequently misdiagnosed lesions. Endometrial polyps are frequently diagnosed as hyperplasia due to poor conduction problems and excessive curettage.4,6,7
Treatment Options
Although there is no bona fide treatment for hyperplasia endometrial, most current guidelines recommend hormone therapies (including use of progestin, levonorgestrel-releasing intrauterine device (IUD) Mirena, gonadotropin-releasing hormone (GnRH) or its analogues or their combination) or surgical treatment (Figure 3). The selection criteria for treatment options are based on patient age, health, the presence of cytologic atypia and fertility status. Hyperplasia endometrial without atypia responds well to progestins. Hormone therapy is also recommended for women whose general health prevents them from tolerating surgery due to coexisting medical conditions. However, women with atypical hyperplasia or persistent hyperplasia without atypia that are symptomatic (abnormal uterine bleeding) are treated with hysterectomy. Among women hoping for childbirth, hyperplasia treatment is challenging, demanding conservative treatment regardless of whether the hyperplasia is with or without atypia.
Atypical hyperplasia is a precancerous lesion and requires a different treatment approach than other types of hyperplasia and adenocarcinoma.7 Hyperplasia without atypia responds very well to progestagens, whereas hyperplasia with atypia requires a hysterectomy.7,8
Regardless of the type of hyperplasia, it should be considered as a warning for changes in the endometrium that do not have cycles, which makes it susceptible to cancerous changes.8 Only the presence of hyperplasia is not a criterion for surgery. Prompt treatment can help us to reverse the injury and avoid radical surgeries.8
Several investigators have observed beneficial effects when treating hyperplasia and carcinoma with progesterone.8
Treatment and management are aimed at preventing the development and progression of endometrial malignancy, to rule out coexisting endometrial malignancy and offer a treatment plan that is tailored to the patient’s need.9
The duration of treatment should last less than six months with progestogens with the following doses medroxyprogesterone of 10-20 mg every day or norethisterone 10-15 mg every day, recommending surveillance with endometrial biopsy at an interval of 6-months, important to take into account that to discharge the patient, two consecutive negative biopsies must be obtained in the semester.8,9
To consider hysterectomy, some criteria should be taken into account that atypical hyperplasia develops during treatment, that there is no resolution after 12-months of treatment, a relapse of endometrial hyperplasia, and a patient who completely refuses surveillance and follow-up.9,10
The role of transvaginal ultrasound is the primary imaging study where an increase in endometrial thickness will be shown, irregular, and biopsy is recommended. Some reviews suggest that when the endometrium measures 3-4 mm, the chance of cancer is reduced by 1%, and no sampling is required.10 Additional evaluations will be indicated in women treated with tamoxifen with ultrasound, hysteroscopy, or biopsy if the endometrial thickness is greater than 4 mm or cannot be visualized correctly. Asymptomatic postmenopausal women who are incidentally diagnosed with a thickness greater than 4 mm do not need routine evaluation.11 In our patient’s case, it was decided to send to surgical treatment since she comes from a distant place with little access to medical resources, few financial resources, and satisfying parity; most of these cases have been observed in that they quit for medical follow-up.
CONCLUSION
Endometrial hyperplasia frequently presents with menorrhagia. Histopathology, in conjunction with the clinic, is essential for the final diagnosis. Clinical and surgical treatment was considered according to the needs of the patient.
CONSENT
Authors have received written, informed consent from the patient.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.








