DEFINITION OF PROTEINURIA
Normal total urine protein excretion is approximately <150 mg/day and any value above this is considered proteinuria.1 In majority of cases, the proteinuria that is excreted inappropriately is albumin. Normal daily albumin excretion is<30 mg/day with the mean being 5-10 mg/day. Standard urine dipstick test only detects albumin once level is significantly elevated, usually at 300 mg/day (Table 1).2,3
| Table 1. Normal Levels for Albumin and Protein Excretion in Urine Studies4 |
|
24 h Albumin (mg/24 h)
|
Albumin/Creatinine Ratio (mg/g) |
24 h Protein (mg/24 h) |
Dipstick/Protein Reagent Strip
|
| Normal to mildly increased albuminuria |
5-10
|
<30 |
<150 |
Negative
|
| Moderately increased albuminuria |
30-300
|
30-300 |
150-500 |
Trace to 1+
|
| Severely increased albuminuria |
>300
|
>300 |
>500 |
1+ to 4+
|
| Nephrotic range proteinuria |
>3500
|
>3500 |
>3500 |
4+
|
| Nephritic range proteinuria |
<1000-2000
|
<1000-2000 |
<2000 |
1+ to 3+
|
PROTEINURIA IN CLINICAL PRACTICE
Proteinuria has many clinical implications. It is used for the diagnosis, staging, progression, and treatment response of chronic kidney disease due to diabetic and non-diabetic etiologies. Albuminuria is primarily used to evaluate diabetic kidney disease. Proteinuria is also an independent marker of cardiovascular disease and mortality. Persistent proteinuria indicative of renal disease has been associated with increased atherosclerosis and left ventricular abnormalities.5,6,7,8
PATHOPHYSIOLOGY OF PROTEINURIA
There are four mechanisms by which proteinuria may occur: glomerular, tubular, overflow and post-renal. Each mechanism tends to result in a specific degree of proteinuria (Table 2).
| Table 2. Mechanisms of Proteinuria |
|
Type
|
Pathophysiology |
Common Causes |
Daily Protein Urinary Excretion (grams)
|
| Glomerular |
Increased glomerular capillary permeability to protein |
Primary: Minimal change disease, Focal segmental glomerulonephritis
Secondary: DM nephropathy, collagen vascular disease, infections |
>0.15 to >3.5
|
| Tubular |
Decreased proximal tubular reabsorption of proteins in glomerular filtrate |
Hypertensive nephrosclerosis, sickle cell disease, uric acid or light chain nephropathy, drugs, toxins or heavy metals |
0.15 to 2.0
|
| Overflow |
Increase production of low molecular weight proteins |
Hemoglobinuria, myoglobinuria, multiple myeloma, acute leukemia, amyloidosis, light chain disease |
0.15 to 2.0
|
| Post-renal |
Inflammation of urinary tract |
Urinary tract infection, nephrolithiasis, uroepithelial tumors |
0.15 to 1.0
|
Glomerular Proteinuria
This is the most common cause of pathological proteinuria and results from increase glomerular filtration of plasma proteins (primarily albumin) due to altered glomerular capillary permeability.
Normally, the charge and molecular size selectivity of the glomerular capillary wall prevents albumin, globulins and high molecular weight (HMW) proteins from crossing. Capillary endothelial cells and glomerular basement membrane have a negative charge owing to the presence of polyanions such as heparin sulfate proteoglycans.5,9 These polyanions repel other anions like albumin.
The glomerular capillary walls also have functional pores through the glomerular basement membrane that traps most large proteins with a molecular size of >100 kDa. Low molecular weight (LMW) proteins <20 kDa are freely filtered. Therefore, measuring urine albumin is more sensitive for detecting changes in glomerular permeability.5,9
Tubular Proteinuria
In tubulointerstitial disease, there is impaired reabsorption at the proximal tubule due to defects in epithelial cells.
Usually, low molecular proteins are easily filtered through the glomerulus then nearly fully reabsorbed in the proximal tubule with only <150 mg/day of protein excreted in urine.1 These proteins include β-2 microglobulin, immunoglobulin light chains, apoproteins and polypeptides. Defects in the tubuloepithelial cells result in increased tubular excretion of these proteins. Tubular proteinuria is usually missed clinically as these non-albumin proteins are not detected by dipstick.1,2
Overflow Proteinuria
Increase production of normal or abnormal proteins creates a filtered load that surpasses the resorptive capacity of the tubule. This usually leads to increased urinary excretion of low molecular proteins.2,10
For instance, with plasma cell dyscrasias such as multiple myeloma, there are an overabundance of immunoglobulin (Ig) light chains. This increased production overwhelms the tubule’s resorptive ability leading to overflow in the urine. Persistent overflow can lead to damage of the tubular cells, which results in tubular proteinuria.1,5
Post-Renal Proteinuria
Inflammation of the urinary tract, for example in acute urinary tract infection, can lead to an increase excretion of small amounts of non-albumin proteins (often IgA or IgG).2 The mechanism of this remains unclear. Leukocyturia is normally seen in these patients.
ETIOLOGY
Broadly, proteinuria can be classified into isolated and non-isolated causes. Isolated proteinuria is usually benign, non-sustained and associated with urine levels <1 g/day.2,4 Patients are usually asymptomatic with an incidental finding of proteinuria and have no prior history of hypertension and diabetes. There are two main types of isolated proteinuria, transient and orthostatic (Table 3).
| Table 3. Comparing Isolated Causes of Proteinuria |
|
Transient
|
Orthostatic
|
| Clinical findings/ associations |
Congestive heart failure (CHF) Sleep apnea Obesity Infection/Fever Strenuous exercise Emotional stress |
Tall, thin adolescents Adults younger than 30-years Severe lordosis |
| Protein level |
<1 g/day |
Usually <1 g/day |
| Urine/Lab studies |
Normal urine sediment, no renal impairment |
Normal urine sediment, no renal impairment Split urine sample: abnormal protein excretion only in the upright sample. |
| Clinical Course |
Benign. Incidental finding. Resolves within days. |
May be persistent but remains benign. If persistent, yearly monitoring required. |
Causes of transient proteinuria are thought to be mediated by angiotensin 2 or norepinephrine-induced alterations of glomerular permeability. In addition, with strenuous exercise, protein excretion can exceed up to 2 g/day.2,4,11,12,13,14 Fortunately, there is no morbidity or mortality associated with this type of proteinuria and repeat dipstick testing is negative.
Orthostatic proteinuria occurs only in the upright position. Although the exact mechanism remains unclear, alteration in glomerular hemodynamics and neurohumoral activation may be contributing factors.2,4 In contrast to transient proteinuria, proteinuria may be persistent on repeated qualitative testing. A split urine sample, where protein excretion is quantified on an upright (during the day) and recumbent (nighttime) sample, is necessary to make the diagnosis. Despite its persistence, orthostatic proteinuria is a benign condition.15,16,17
In contrast, non-isolated proteinuria is usually due to glomerular or systemic diseases and associated with urine levels >1-2 g/day.4 It can be further classified into nephrotic and nephritic proteinuria. Nephrotic proteinuria is protein excretion >3500 mg/24 h or a urine protein-creatinine ratio of >3500 mg/g. If this level of proteinuria is associated with hypoalbuminemia, edema and hyperlipidemia, nephrotic syndrome is diagnosed.18 On the other hand, nephritic syndrome is associated with glomerular inflammation that results in renal failure, hypertension, hematuria, non-nephrotic range proteinuria and dysmorphic erythrocytes on microscopic analysis (Tables 4 and 5).18,19,20
| Table 4. Common causes of Nephrotic Syndrome18 |
|
Condition
|
Clinical Clues |
Secondary Causes
|
| Minimal change disease |
Young adults/ paediatric population |
Lymphoma NSAIDs |
| Membranous
glomerulopathy |
Caucasian adults History of cancer Renal vein thrombosis |
Systemic lupus erythematosus (SLE) Solid tumors |
| *Membranoproliferative glomerulonephritis (MPGN) |
Low complements |
Infection (Hepatitis C) SLE Monoclonal gammopathy |
| Focal segmental glomerulosclerosis |
African Americans APOL1 & MYH9 gene |
Morbid obesity Human immunodeficiency virus infection (HIV) Heroin |
| Diabetic nephropathy |
Adult population, most common overall cause |
– |
| *Also presents with nephritic syndrome NSAID: Nonsteroidal anti-inflammatory drugs; |
| Table 5. Common Causes of Nephritic Syndrome18 |
|
Pathology
|
C3 and C4 |
Specific Diseases |
Workup |
Clinical Associations/Pearls
|
| Anti-glomerular basement membrane (GBM) antibodies |
Normal |
Anti-GBM antibody disease |
Kidney biopsy: linear deposition of immunoglobulin |
Goodpasture’s syndrome if pulmonary hemorrhage |
| Pauci-immune glomerulonephritis (GN) |
Normal |
Eosinophilic granulomatosis with polyangiitis Granulomatosis with polyangiitis (GPA) Microscopic polyangiitis (MPA) |
Kidney biopsy: absent or minimal staining with immunoglobulin |
GPA and MPA are ANCA positive > 80% of time: PR3-ANCA and MPO-ANCA respectively |
| Immune complex deposition |
Low |
Cryoglobulinemia *IgA nephropathy *IgA vasculitis (Henoch-Schonlein Purpura) Infection-related GN Lupus Nephritis Membranoproliferative (MPGN) |
ANA and anti-double stranded antibodies Antistreptolysin O and anti DNasetiters |
Cryoglobulinemia: associated with hepatitis C virus (HCV) infection IgA nephropathy: most common primary glomerular disease. IgA vasculitis: tetrad of rash, arthralgia, abdominal pain, and kidneydisease. Poststreptococcal GN: occur after latent period of 1-6 weeks. |
| *normal complement |
METHODS FOR DETECTING AND MEASURING PROTEINURIA
Proteinuria can be detected by either semi-quantitative or quantitative tests. Semi-quantitative tests are usually the primary means by which proteinuria is first detected. These are of two types: urine dipstick, which is more commonly used, and sulfosalicyclic acid test (SSA).
Dipstick Testing
A urine dipstick test should preferably be done on a mid-stream catch for males and clean catch sample for females to avoid contamination and improve the accuracy of test. A positive test can be reported as trace to 4+. The dipstick test is easy, quick and cheap to perform. Importantly, the dipstick only detects albumin. Moreover, albumin levels must be severely increased, i.e. macroalbuminuria (>300 mg/day) before a positive result is obtained.1,5
Consequently, there are limitations to this test. Firstly, the test is insensitive for detecting other proteins, including low molecular weight proteins such as the immunoglobulin light chains.1 Secondly, it is not useful for detecting urinary protein levels <300 mg/day, i.e microalbuminuria.2 Lastly, false test results can occur. False positive results can occur if urine pH is >7.0, if the sample is very concentrated or contaminated with blood or antiseptics (such as chlorhexidine). Likewise, false negative results can occur if urine is dilute, if there is only mild loss in protein or if the proteinuria is not due to albumin.22,23,24,25 Despite these limitations, dipstick testing remains the most common method for detecting proteinuria, as most causes of proteinuria is due to albuminuria.26
Sulfosalicyclic Acid Test
If there is high clinical suspicion for non-albumin proteinuria, an SSA test should be performed.27 For instance, a patient with acute kidney injury, benign urinalysis and negative dipstick. During this test, eight drops of 20% solution of SSA are added to 10 ml of urine and results are recorded as 0 to 4+.2,5
This test detects all types of proteins, including immunoglobulin light chains, at levels as low as 5-10 mg/dL. A positive test is based on the resulting turbidity of the urine and ranges from 0 (no turbidity; omg/dL) to 4+ (flocculent precipitate; >500 mg/dL).1,27 Likewise, with dipstick testing, false positives can occur in the presence of hematuria or highly concentrated urine. Other causes include iodinated radiocontrast agents and medications such as penicillin, cephalosporins and sulfisoxazole.23,24,25,28 Although SSA has a higher sensitivity at detecting proteinuria of all types at lower levels, it is rarely used in routine practice.
Quantitative Tests
When the above tests are abnormal, a quantitative test is warranted. The two options are a timed 24-hour urine collection or a spot protein/creatinine sample.
Timed 24-hour urine collection
The 24-hour urine collection remains the gold standard test as there is variation in protein excretion with circadian rhythm.5 The normal value is less than 150 mg/day. In order to determine if the result is reliable, the 24-hour urine creatinine is measured and compared to the expected urine creatinine per kilogram of lean body mass. Men excrete 19-26 mg of creatinine/kg/day and women excrete 14-21 mg/kg/day. Of note, these values vary with muscle mass and weight; for example, the more muscle mass a patient has, the more creatinine excretion.2,5,29,30 The major limitation with this test is the collection compliance: it is cumbersome for patients and it is often either over- or under collected.1
Spot Urine Protein/Creatine Ratio (UPCR)
Due to the limitations of the 24-hour urine collection, the spot UPCR test has also been supported by Kidney Disease: Improving Global Outcomes (KDIGO) as an appropriate method to measure proteinuria. In this test, both the protein and creatinine concentrations are measured (in mg/dL), and the ratio is calculated (mg/ mg). This ratio has been shown to correlate well with the daily protein excretion (g/day). An UPCR of 0.2 or less is normal versus a ratio of 3.5 or greater signifies nephrotic range.2,5,31,32,33,34
The main advantages this test offers are the ease of collection and good correlation with daily protein excretion at the population level. The major limitation of this test is that it functions based on the concept that there are steady levels of creatinine and protein excretion daily. However, as explained earlier, excretion follows a circadian rhythm and varies depending on the proportion of muscle mass.35,36,37 To offset this variation, the patient should collect all samples at about the same time of day.
APPROACH TO PROTEINURIA
When a patient presents with proteinuria, the first important step is to rule out causes for a false positive test.5 Once we believe that the patient has true proteinuria, then a step wise approach should be taken that involves careful history, physical exam and vitals, labs and urine studies. Depending on these results, radiologic studies, serology and renal biopsy may be warranted.
In general, if proteinuria is associated with abnormal urine sediment analysis, impaired renal function or history and physical findings suggestive of systemic or glomerular pathology, then a quantitative urine test should be performed to rule out nephrotic or nephritic syndromes.1,4,19
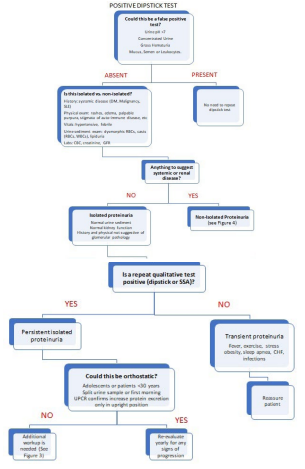
On the other hand, if the patient has an isolated proteinuria, it can either persist (in which case orthostatic proteinuria should be ruled out first) or can spontaneously resolve indicating transient causes.5,17 Renal ultrasound is recommended to rule out structural causes in cases of persistent proteinuria. A renal biopsy is indicated if: 1) proteinuria persists and is greater than 1 g/day with no diagnosis of orthostatic proteinuria; 2) patient develops non-isolated findings (hematuria, active urine sediment, hypertension, low glomerular filtration rate (GFR)) or 3) nephrotic range proteinuria.2,5,37,38 An active urine sediment is generally identified by the presence of >5 red blood cells (RBCs) and >5 white blood cells (WBCs) per high power field (hpf) and/or the presence of cellular casts. Findings may include dysmorphic RBCs, WBCs (including eosinophils) and casts (RBC, WBC, waxy and broad) and can indicate the pathology. For instance, dysmorphic RBCs and RBC casts usually indicate glomerular injury, while the presence of WBC casts is usually indicative of interstitial and/or tubular damage. Lipid droplets or fatty casts are commonly associated with nephrotic syndrome (Figures 1, 2 and 3).24
Figure 1. Algorithm for the Approach to Proteinuria
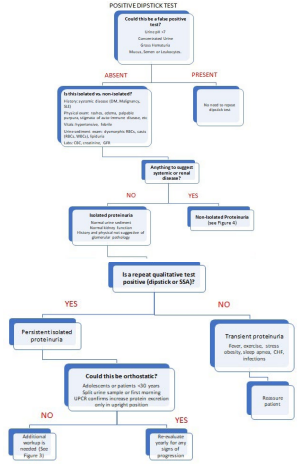
Figure 2. Algorithm for the Approach to Persistent Proteinuria that is not Due to Orthostatic Proteinuria
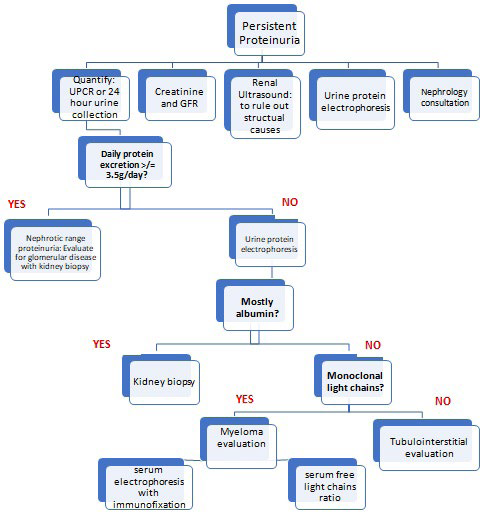
Figure 3. Algorithm for the Approach to Non-isolated Proteinuria
CONCLUSION
Proteinuria is a common finding that physicians will encounter. Although commonly transient and benign, proteinuria may also indicate significant renal disease, such as a glomerulopathy, tubulointerstitial nephritis or vasculitis. Glomerular diseases are the third leading cause of end-stage kidney disease (ESKD) in the United States, accounting for approximately 10,000 incident cases of ESKD per year, third to diabetes mellitus and hypertension.39 In addition, albuminuria is frequently used as an index for kidney function to detect early stages of chronic kidney disease (CKD), which can progress to ESKD.3
Unfortunately, proteinuria can be commonly missed. One of the factors that may contribute to late diagnosis is the sensitivity and preferentiality of the urine dipstick test, i.e. it primarily detects albumin at severely increased levels. Consequently, when proteinuria is found incidentally, it should not be ignored but rather, at the very least, causes of false-positive results should be ruled out.2,5 If these causes are absent, then there should be a systematic approach to managing these patients. In order to provide an appropriate assessment of patients with proteinuria, physicians must understand the physiological aspects of protein handling by the kidney and recognize the clinical and lab findings that suggest renal pathology. Proteinuria due to clinically significant renal disease is persistent and should be evaluated further. When proteinuria is due to glomerular/renal disease, early diagnosis is critical to prevent further renal damage.5
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.








