INTRODUCTION
Poultry production has become an important part of economic activity in many countries. In large-scale intensive production, poultry production is exposed to many stressful conditions and diseases that result in serious economic losses. Currently, prevention measures using antimicrobial agents have been questioned due to the evolution of antimicrobial resistance among pathogenic bacteria. Accordingly, probiotics are being considered as the best option to fill the gap and already used by some farmers in preference to antibiotics.1,2
Probiotics were first coined by Lilly and Stillwell in 1965 and derived from the Greek word, meaning ‘for life’ and in contrast to antibiotic, probiotics defined as “substances secreted by a microorganism that stimulates the growth of another”. Later in 1989, the definition was modified by Fuller as “live microbial cultures which beneficially affect the host by improving its intestinal microbial balance”.2,3
At the end of 20th century, the concept of probiotics evolved from a hypothesis first proposed through the Russian scientist and Nobel Laureate, Elie Metchnikoff, who cautioned that the lengthy, healthy existence of Bulgarian peasants; resulted from their consumption of fermented milk products. He believed that consumption of the fermenting Lactobacillus positively influenced the microflora of the gut, decreasing the toxic microbial activity of the pathogenic bacteria population.4,5
A probiotic also referred to as direct-fed microbial, is a culture of a single bacterial strain, or a mixture of different strains, that can be fed to an animal to improve its health. A variety of different types of bacteria, and in some cases even undefined cultures, have been tested as probiotics in poultry. The aim of many studies involving direct-fed microbials has been to exclude the colonization of pathogens in the gastrointestinal tract of poultry.6,7,8
Probiotics can prevent pathogen colonization of the gut and reduce the incidence or relieve the signs and symptoms of numerous diseases due to dysregulated immune responses. Probiotics seem to function by influencing both intestinal epithelial and immune cells of the gut, but the details of these effects are still being unraveled. So, probiotics enhance the host immune system and used to prevent diseases. The beneficial effects of probiotics can vary between strains so, the selection of the most suitable ones will be crucial for their use in the prevention or treatment of specific diseases. In order for a potential probiotic strain to exert to its beneficial effect, probiotics need to be delivered to the desired sites in an active and viable form. The viability and activity of probiotics in the products have been frequently cited as a prerequisite for achieving numerous beneficial health benefits.9,10
SOURCES OF PROBIOTICS
Microorganisms Used as Probiotics
The success of probiotics depends upon the survival and stability of the probiotics, the strain, the age, host specificity of the strain, dose rate, physiological and nutritional status of the bird genetic make-up of the host.11In contrast to the crop, proventriculus, and gizzard, the small intestine contains a large number of facultative anaerobes such as Lactobacillus, Streptococci, and anaerobes like Bacteroides and Bifidobacterium species. Probiotics colonize three different regions within the gastrointestinal tract (GIT); enterocyte, cecal and colonic epithelium.3,12,13
Lactobacillus, Bifidobacterium, Enterococcus are among the most commonly used Genera of probiotic microorganisms in human nutrition. whereas yeast especially Saccharomyces cerevisiae plays a major role in ruminants; while Bacillus, Enterococcus and Lactobacillus are more likely to be efficient in pigs and poultry.14,15 Some of the important strains of microorganisms considered as probiotics are listed in Table1.
| Table 1. Strains of Microorganisms Frequently Used as Probiotics in Poultry |
|
Genera of Probiotic Microorganisms
|
Strain of Microorganisms
|
| Lactobacillus species |
L. acidophilus, L. casei, L. crispatus, L. gasseri, L. fermentum, L. johnsonii, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, L. bulgaricus |
| Bifidobacterium species |
Bifidobacterium bifidum, B. breve, B. lactis, B. longum, B. infantis, B. adolescentis, B. animalis |
| Enterococcus species and Lactic acid bacteria |
Enterococcus faecalis, E. faecium, Lactococcus lactis, Leuconostoc mesenteroides, Pediococcus acidilactici, Streptococcus thermophilus |
| Non-lactic acid bacteria |
Bacillus cereus, Bacillus subtilis, Escherichia coli strain nissle, Propionibacterium freudenreichii, Aspergillus oryzae, Saccharomyces acidophilum, Saccharomyces boulardii, Saccharomyces cerevisiae |
| Source:16,17 |
Other Sources of Probiotics
Yoghurt and other fermented milk products such as cultured buttermilk and cheese are among dominant foods used as sources of probiotics that provide a relatively low pH environment that facilitates the survival of the probiotic bacteria.18Lactic acid bacteria, Bifidobacteria and other microorganisms isolated from fermented milk products. Spontaneous milk fermentation has a long history in different regions of Mongolia and Africa, and the use of beneficial microorganisms in fermented dairy products has been practiced for many generations. These traditional fermented milks contain complex compositions of Lactic acid bacteria species and therefore provides a useful source of probiotic strains.19
In addition, probiotics can also be found in non-dairy fermented substrates such as soy-based products, cereals, legumes, cabbage, maize, pearl millet and sorghum.16,18 The other sources of probiotics include breast milk, the human gastrointestinal tract, and the guts of several animal species, including pigs, rats, and even poultry. Recent researches were performed to assess traditional fermented products for their potential capacity as natural resources of probiotic bacteria. Generally, most of the microorganisms isolated from fermented products belong to the Lactobacillus genus.20,21,22
PRINCIPLES FOR SELECTION OF PROBIOTICS IN THE POULTRY INDUSTRY
During the selection of probiotics strain, safety accesses must be kept in mind regarding production relating to the technological aspects, application, survival, and colonization in the host and their health benefits.23
Resistance to Low pH and Bile Salts
Acid tolerance is one of the general criteria that is considered during the selection of potential probiotic strains to secure their viability and feasibility.24 The survival of ingested probiotics in different parts of the gastrointestinal tract varies with the strain. Some strains are rapidly killed in the stomach while others, such as strains of Bifidobacteria or Lactobacillus acidophilus, can pass through the whole gut at very high concentrations. Numerous in vitro and in vivo studies have demonstrated that probiotics organisms can survive in the gastric transit where the cells as exposed to acidic environment.5,25
Probiotics display enormously variable resistance to acid and bile salts and this feature of probiotics is each species and strain-dependent. Procedure to determine the bile resistance: first, bacterial cells are suspended in Man, Rogosa and Sharpe (MRS) broth (originally developed in 1960 by de MRS) with 0.2% and 0.4% of bile salts. Then the broth will pour into three tubes. One as a control incubate in MRS broth without bile salts and other tubes contains 0.2% and 0.4% bile salts, respectively kept for incubation. Finally, look for their optical density by spectrophotometer at 540 nm.6,25
Adherence to Intestinal Epithelial Cells
The adherence of probiotic to intestinal mucus and epithelial cells to colonizes intestinal epithelium have long been considering as one of the most important selection criteria for probiotic microorganisms. Adhesion to the intestinal mucosa may additionally prevent the probiotic cells being washed out and consequently, enabling temporary colonization, immune modulation and competitive exclusions of pathogens. The probiotic strain must adhere to the intestinal wall, colonize and multiply in order to produce enzymes, lactic acids, vitamins, and natural antibiotics.2,4 During intestinal infections, the adhesion of pathogenic bacteria to mucosal surfaces and disruption of the intestinal microbiota is anticipated. Accordingly, the probiotic bacteria might play protective as well as defensive roles through adhesion and colonization of the mucosal surfaces, effectively competing with pathogens for binding sites and nutrients and immune stimulation.26,27
Antimicrobial Activity of Probiotics
The probiotic strain should be capable of producing antimicrobial substances is most important in developing the probiotic supplement and probiotic-rich foods. When administered in adequate amounts, probiotics confer health benefits to the host.24,28 Probiotics might act antimicrobial activity against pathogens through a variety of mechanisms, including the production of antimicrobial substances, competition with pathogens for nutrients and adhesion sites and stimulation of the immune system. Lactic acid bacteria produce several metabolic compounds such as organic acids, fatty acids, hydrogen peroxide, and diacetyl that have antimicrobial activity. Yet, bacteriocins or proteinaceous substances with specific inhibitory activity against closely related species are most studied.29,30
MECHANISMS OF PROBIOTICS ACTION
Enhancement of Epithelial Barrier Function
Probiotics are able to influence many of the components of epithelial barrier function by decreasing apoptosis of intestinal cells. Lactobacillus rhamnosus GG was able to prevent cytokine-induced apoptosis in intestinal epithelial cell models by inhibiting tumor necrosis factor (TNF).31 Integral to the gut barrier defense is mucus which is composed of mucins, which are secreted from the goblet cells. Mucin polymerization provides the structural foundation of the mucus, granting protection from pathogens, enzymes, toxins, dehydration, and abrasion. Some of the probiotics like lactobacilli, for instance, have been shown to modulate the regulation of several genes encoding adherence junction proteins such as E-cadherin and β-catenin in T84 epithelial cells.32,33
Competition for Adherence
Probiotic competition for adhesion sites on the intestinal epithelium can prevent the formation of colonies of pathogenic bacteria. Probiotic microorganisms compete with invading pathogens for binding sites to epithelial cells and the overlying mucus layer in a strain-unique manner. Once the probiotic adheres to the cell, different biological activities take place, which primarily include the release of cytokines and chemokines. Then, they exert their secondary activity such as stimulation of mucosal and systemic host immunity. For instance, Saccharomyces boulardii, a non-lactic acid bacterium, secretes a heat-labile factor that has shown to be responsible for the decreased bacterial adherence.34,35,36
Competitive Exclusion of Pathogenic Microorganisms
Probiotic bacteria are able to exclude or reduce the growth of pathogens by colonization of favorable sites of adhesion such as the intestinal villus and colonic crypts, or excretion of the mucins (MUC2 and MUC3) from goblet cells which inhibits the adherence of enteropathogenic bacteria. Lactic acid bacteria produce several metabolic compounds such as acetic acid and lactic acid that induces a hostile microenvironment by the reducing of the pH of the gut below than what’s critical for the survival of pathogenic bacteria. In addition, Wang et al37 showed that lactic acid could even completely inhibit growth of pathogens inclusive of E. coli, Salmonella and L. monocytogenes. The others include physical blocking of available bacterial receptor sites32,38,39; compete with pathogenic bacteria for essential nutrients and energy source; secretion of antimicrobial substances and release of selective gut protective metabolites like arginine, glutamine, short-chain fatty acids and conjugated linoleic acids.35,40
Production of Antimicrobial Substances
Probiotics have been shown to suppress pathogen growth through the release of a variety of antimicrobial factors like defensins, bacteriocins and short-chain fatty acids, such as lactic and acetic acids, which reduce the pH of the lumen. Short-chain fatty acids can disrupt the outer membranes of gram-negative pathogens causing inhibition of pathogen growth.41,42,43 Bacteriocins are antimicrobial compounds produced by gram-positive bacteria usually the lactic acid bacteria include lactacin B from Lactobacillus acidophilus, plantaricin from L. plantarum and nisin from Lactococcus lactis. These have a narrow activity spectrum and act only against closely related bacteria, but some bacteriocins are also active against food-borne pathogens. The common mechanisms of bacteriocin-mediated killing include the destruction of target cells by pore formation and/or inhibition of cell wall synthesis.39,40
Modulation of the Immune System
Probiotics have the capability to enhance the immune system by increasing the phagocytic capacity of macrophages, enhancing natural killer cell activity, stimulating immunoglobulin A (IgA) production, and modulation of cytokine production.44,45
Interference with Quorum Sensing Signaling Molecules
Quorum sensing or auto-inducers are chemical signaling molecules used for bacterial communication with each other as well as with their surrounding environment. This phenomenon of communication is one of its characteristics that control the gene expression. Probiotic bacteria such as lactobacillus, bifidobacterium and Bacillus cereus strains degrade the auto-inducers of pathogenic bacteria by enzymatic secretion or production of auto-inducer antagonists and thereby control the virulence gene expression in pathogenic bacteria.32,38
APPLICATION OF PROBIOTICS IN POULTRY PRODUCTION
Probiotics have been reported to increase feed efficiency and productivity of laying hens with an improvement in egg quality by decreased yolk cholesterol level, improved shell thickness and egg weight. Similarly, probiotics such as Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida and Saccharomyces have shown beneficial effect on broiler performance species with evidence of increased resistance of chickens to Salmonella, Escherichia coli or Clostridium perfringens infections.9,46
Role against Pathogens Infection
Probiotics have a great role in the stimulation of protective immune response and help to suppress the growth of potential gut pathogens in poultry. The inhibition of pathogen by probiotics is suggested to occur via competition for adherence sites on the intestinal wall and nutrient as well as the production of antimicrobial compounds.10,47 Probiotics such as lactic acid bacteria have been widely known for its importance in exerting inhibitory and antagonistic effects against pathogenic bacteria. Numerous studies have been reported that probiotics can exert antimicrobial effect against pathogenic bacteria via the production of metabolites.2,43,48
Intestinal colonization with probiotic Lactobacillus strains has been demonstrated to have a preventive function against Salmonella enterica serovar enteritidis infection in chicken.33,49 On the other hand, bacteriocins with antimicrobial properties have been reported to show promising growth inhibition potential against intestinal pathogenic bacteria. Bacteriocins derived from Lactobacillus salivarius exhibit strong antagonistic activity against Campylobacter jejuni and Gram-positive bacteria Pilasombut et al,50 reported that oral inoculation of Bacillus subtilis spores could reduce intestinal colonization of E. coli O78: K80 in chickens.50
Role on stimulation of Immune Responses
According to Kabir et al,7 the dynamics of probiotics related to immune responses demonstrated that antibody production was elevated in broilers after fed with probiotics containing Lactobacillus. The modulation of immune responses by probiotics is also apparently observed in broilers exposed to stress conditions. Lactobacillus-based probiotics administration was observed to improve heat-stress related problems in broilers which are accompanied by improved antibody production as compared to controls. Supplementation of probiotic Lactobacillus in broilers’ diet revealed that probiotic could enhance intestinal immunity against coccidiosis by altering the population of intestinal intraepithelial lymphocyte expressing surface markers cluster of differentiation 4 (CD4).51,52
Probiotics have also been suggested to augment Toll-like receptor signaling in which Toll-like receptor plays a crucial role in the activation of T-cells in the intestinal immune system. A recent study showed that probiotic products consists of Lactobacillus fermentum and Saccharomyces cerevisiae increased the level of messenger ribonucleic acid (mRNA) expression of Toll-like receptors-2 (TLR-2) and 4 in the foregut of the chickens compared to those administered with control diet and antibiotic.48 Furthermore, basal diet supplemented with probiotics mixture containing Lactobacillus acidophilus, Lactobacillus casei, Enterococcus faecium, and Bifidobacterium thermophilus elevated the concentration of IgG and IgM levels in turkeys and the enhancement of the immunoglobulins level have been proposed to contribute to more positive growth performance, production and resistance to diseases.53
Effects on Intestinal Morphology
Several studies have been carried out to assess the effects of probiotic administration on the histomorphology of the intestine. According to these studies, dietary treatment with probiotic Lactobacillus species such as Lactobacillus sakei Probio-65 was reported to influence the villi height and crypt depth in the small intestine especially jejunum of broilers. Probiotics are proposed to increase the length of villi by activating cell mitosis and induce gut epithelial-cell proliferation.39,48,54 Increased villi height by probiotics is beneficial to the broilers as the increased surface area of the villi enhanced the absorption of nutrients. It has been suggested that alteration in villi length and crypt depth may lead to poor nutrient absorption, digestive enzymes secretion in the GI tract and eventually lower growth performance in broilers.8,55,56
Pelicano et al57 has described that villi in jejunum occur in zig-zag form, resembling wave pattern. It was suggested that the formation of villi in the wave pattern enables better nutrient absorption than villi arranged in parallel or randomly positioned. Zigzag flux in the small intestine permits food to take a longer passage through the alimentary canal compared to the straight flux, and improve the contact between the nutrients and the absorption surface of the intestinal epithelium. Probiotic such as Lactobacillus sakei Probio-65 promoted waved-like arrangement of jejunum villi in broilers (Figure 1).
Figure 1. Scanning Electron Microscopy of Jejunal Villi Arrangement in Broiler Chickens Administered with Antibiotics and Probiotics
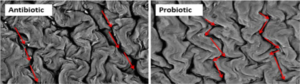
Source: Pelicano et al57
Accordingly, the promotion of gut health by probiotic bacteria further strengthens the potential of probiotics as emerging alternatives to antibiotics as growth promoters in poultry production. Gut condition was well preserved in the presence of probiotics such as Lactobacillus sakei Probio-65, accompanied by healthy development of the intestines of as compared to control broilers that were not fed with probiotics. In contrast to probiotics, antibiotic damaged jejunal villi tip with prevalent shedding at the end of the villi tips (red circle in Figure 2). Injuries of the intestinal walls have been much reported upon the administration of antibiotics, and are very often accompanied by thinning of the intestinal mucus layer and increased depletion of goblet cells.58
Figure 2. Histological Images of Sections from Jejunum Tissue of Broiler Chickens Fed with (A) Antibiotic bacitracin (B) Probiotic Lactobacillus Sakei Probio-65
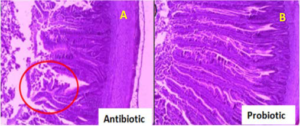
Source: Wlodarska et al58
Role in Growth Performance
The role of probiotics as dietary supplementation and growth performance has been extensively investigated in poultry production. Most studies indicated that probiotics shown great efficacy in promoting animal growth. Lactobacillus inclusion in broilers nutrition also resulted in a higher broiler productivity index, which is measured based on daily weight gain, feed efficiency, and mortality. While growth rates of the broilers are improved, the Lactobacillus administration reduced the mortality of the broilers which usually arose from pathogen infections. Moreover, probiotics supplementation to diet improved feed intake, feed efficiency, and carcass yield of broilers.3,59,60
According to recent investigations on the effects of probiotic supplementation on digestive enzymes activity in broiler chickens revealed that the probiotic Bacillus coagulans NJ0516 promotes higher activity of protease and amylase. This finding suggests that the higher activity of the enzymes may lead to better digestibility of protein and starch, which in turn explains better growth in broilers fed with probiotics rather than control basal diet.27 On the other hand, dietary supplementation of probiotic Lactobacillus sporogenes lowered serum level of total cholesterol, lowdensity lipoprotein (LDL) cholesterol, very-low-density lipoprotein (VLDL) cholesterol and triglycerides.2,16,47
Role on Quality Poultry Products
Probiotics increase egg production, improve egg quality and decrease egg contamination. Further, probiotics increase eggshell weight, shell thickness and serum calcium in layers and also diets supplemented with commercial probiotic improves and decreased broken egg ratio in layers. According to Panda et al,47 dietary preparation of Lactobacillus sporogenes at 100 mg (6×108 spores) per kg of diet significantly increased egg production, eggshell strength, shell weight and shell thickness in laying hens without affecting egg weight, specific gravity, and Haugh unit.3,9,61
Probiotics supplementation improves the meat quality in broilers which is recognized all over the world. Intramuscular lipid content is involved in determining meat quality particularly nutrition, tenderness, odor, tastes and flavor characteristics. Greater tendency of higher ratio of unsaturated fatty acids to saturated fatty acids in pectoral and thigh meat of broilers fed with probioticssupplemented diet. The results suggested that the fat in meat was converted into favorable fat in the presence of probiotics, which in turn contributed to meat tenderness. In broilers, improved tenderness was indicated after mixing their diet with probiotic Clostridium butyricum. In contrast to traditional basal diet, the overall organoleptic scores in terms of appearance, texture, juiciness and overall acceptability were higher in probiotic Lactobacillus fed broilers.62,63
Meat in broilers fed with probiotics Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Aspergillus oryzae, Streptococcus faecium and Torulopsis species displayed higher content of moisture, protein, and ash compared to the control.2,64 The results indicated that chicken fed with probiotics has better retention of minerals especially phosphorus, calcium, and nitrogen as well as protein efficiency ratio. According to Hossain et al,65 a higher protein efficiency ratio may subsequently help promote meat yield. Besides, the addition of probiotics increased breast meat absolute and relative weight. Furthermore, the carcass quality of broilers was also reported to be improved by probiotics with lesser occurrence of Salmonella contamination.2,7,9
CONCLUSION
Nowadays, antibiotic resistance and the increase in diseases have posed a great problem in poultry production. Hence, these days the poultry manufacturers’ and owners’ trend is turning towards natural products. Hereafter, probiotics have come under the scanner for its uses as nutritional supplements. Probiotics are a possible device for lowering intestinal infection by disease-causing and foodborne microorganism. Their benefits to human and animal health have been proven in a lot of Scientific Articles. The use of Probiotics in day-to-day medicine in the treatment of gastrointestinal disorders is increasing with the discovery of the beneficial effect of these agents. Lactobacillus and Bifidobacterium are the main probiotic groups; besides, Pediococcus, Bacillus and yeasts are also another probiotic potential. There are several reports on the role of probiotics as a powerful growth promoter, immune modulator, anti-diarrheal effects, increase product quality and other important properties. Inconclusion, the commercial use of probiotics in poultry production has proceeded because essentially no risk is associated with the consumption of well-defined probiotics in foods and many benefits are possible.







